Oklahoma Soil Fertility Handbook
- Jump To:
- Chapter 1 - Soil and Soil Productivity
- Chapter 2 - Essential Plant Nutrient Functions, Soil Reactions and Availability
- Chapter 3 - Problem Soils
- Reclamation
- Reclamation
- Chapter 4 - Determining Fertilizer Needs
- Chapter 5 - Fertilizer Use in Oklahoma
- Chapter 6 - Use of Animal Manure as Fertilizer
- Chapter 7 - Environmental Concerns Associated with Fertilizer Use
- Chapter 8 - Land Application of Drilling Mud
- Chapter 9 - Long-term Fertility Research
- Chapter 11 - Nitrogen-Rich Strips, GreenSeeker Sensor and Sensor-Based Nitrogen Rate Calculator
- Chapter 12 - Laws and Acts Governing the Marketing of Fertilizer, Lime and Soil Amendments in Oklahoma
Chapter 1 - Soil and Soil Productivity
Jason Warren
Soil is perhaps the most important natural resource in Oklahoma. It is important to all, for without soil there would be no life on Earth. Our food and much of our clothing and shelter come from soil. Soil supports the gigantic agricultural system, which is the major contributor to the state’s development and continued prosperity.
Oklahoma has a land area of more than 44 million acres, part of which is covered by
water. The majority, about 41 million acres, is used for production of food and fiber.
This land has an average value of more than $400 per acre or a total value in excess
of $16.4 billion. It is an asset well worth protecting.
Many different kinds of soil occupy this land area. Some soils are extremely productive,
while others are not as productive. Each soil has a set of unique characteristics
that distinguishes it from other soils. These characteristics determine the potential
productivity of the soil.
Soil productivity is a result of how well the soil is able to receive and store moisture
and nutrients, as well as providing a desirable environment for all plant root functions.
What is Soil?
Soil is the unconsolidated mineral and organic material on the immediate surface of the Earth which provides nutrients, moisture and anchorage for land plants.
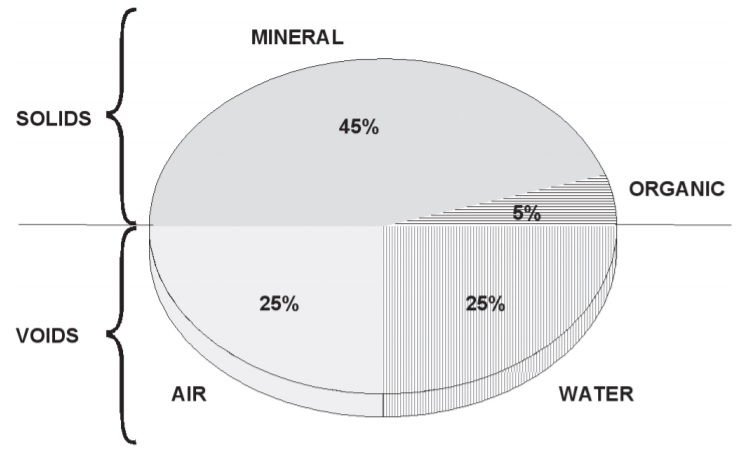
The principal components of soil are mineral material, organic matter, water and air.
These are combined in widely varying amounts in different soils. In a typical loam
soil, solid material and pore space are equally divided on a volume basis, with the
pore space containing nearly equal amounts of water and air. The approximate proportions
are illustrated in Figure 1.1.
How Soils are Formed
The development of soils from parent rock is a long-term process involving physical and chemical weathering along with biological activity. The wide variety of soils and their properties are a function of the soil forming factors including parent material, climate, living organisms, topography and time.
The initial action on the parent rock is largely mechanical-cracking and chipping
due to temperature changes. As the rock is broken, the total surface area exposed
to the atmosphere increases. Chemical action of water, oxygen, carbon dioxide and
various acids further reduce the size of rock fragments and change the chemical composition
of many resulting particles. Finally, the microorganism activity and higher plant
and animal life contribute organic matter to the weathered rock material, and a true
soil begins to form.
Figure 1.1: Volume composition of a desirable surface soil.
Since all of these soil-forming agents are in operation constantly, the process of soil formation is continual. Evidence indicates the soils we depend on today to produce our crops required hundreds or even thousands of years to develop. In this regard, consider soil as a nonrenewable resource measured in terms of man’s life span. Thus, it is very important to protect soils from destructive erosive forces and nutrient depletion, which can rapidly destroy the product of hundreds of years of nature’s work, as well as greatly reduce soil productivity.
Soil Profile
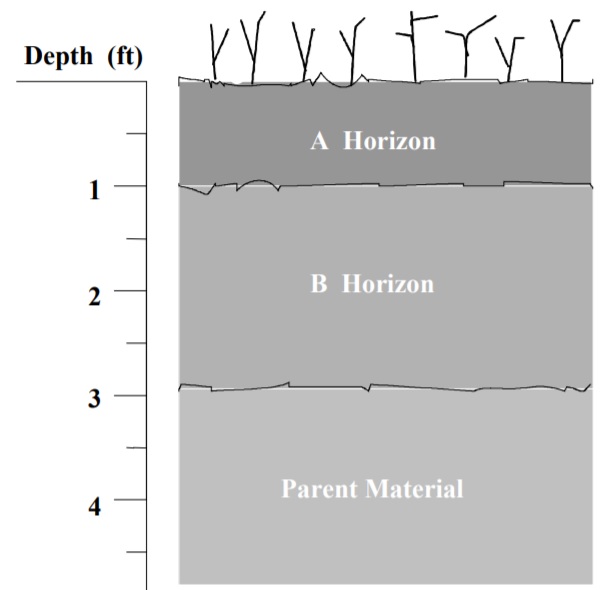
A vertical cross-section through a soil typically represents a layered pattern. This section is called a “profile” and the individual layers are called “horizons.” A typical soil profile is illustrated in Figure 1.2.
Figure 1.2: A typical soil profile.
The uppermost layer includes the surface soil or topsoil and is designated the ‘A’ horizon. This is the layer which is most subject to climatic and biological influence. It usually IS the layer of maximum organic accumulation, has a darker color, and has less clay than subsoil. The majority of plant roots and most of the soil’s fertility are contained in this horizon.
The next successive horizon is called the subsoil or ‘B’ horizon. It is a layer that
commonly accumulates materials that have migrated downward from the surface. Much
of the deposition is clay particles, iron and aluminum oxides, calcium carbonate,
calcium sulfate and possibly other salts. The accumulation of these materials creates
a layer that is normally more compact and has more clay than the surface. This often
leads to restricted movement of moisture and reduced crop yields.
The parent material, or ‘C’ horizon is the least affected by physical, chemical and
biological weathering agents. It is very similar in chemical composition to the original
has formed in its original position by weathering of bedrock is termed “residual;”
or transported if it has been moved to a new location by natural forces. This latter
type is further characterized on the basis of the kind of natural force responsible
for its transportation and deposition. When water is the transporting agent, the parent
materials are referred to as alluvial (stream deposited). This type is especially
important in Oklahoma. These are often the most productive soils for agricultural
crops. Wind-deposited materials are called aeolian.
Climate has a strong influence on soil profile development. Certain characteristics
of soils formed in areas of different climates can be described. For example, soils
in western Oklahoma are drier and tend to be coarser textured, less well developed
and contain more calcium, phosphorus, potassium and other nutrients than do soils
in the humid eastern part of the state.
The soil profile is an important consideration in terms of plant growth. The depth
of the soil, its texture and structure, its chemical nature as well as the soil position
on the landscape and slope of the land largely determine crop production potential.
The potential productivity is vitally important in determining the level of fertilization.
Soil Texture
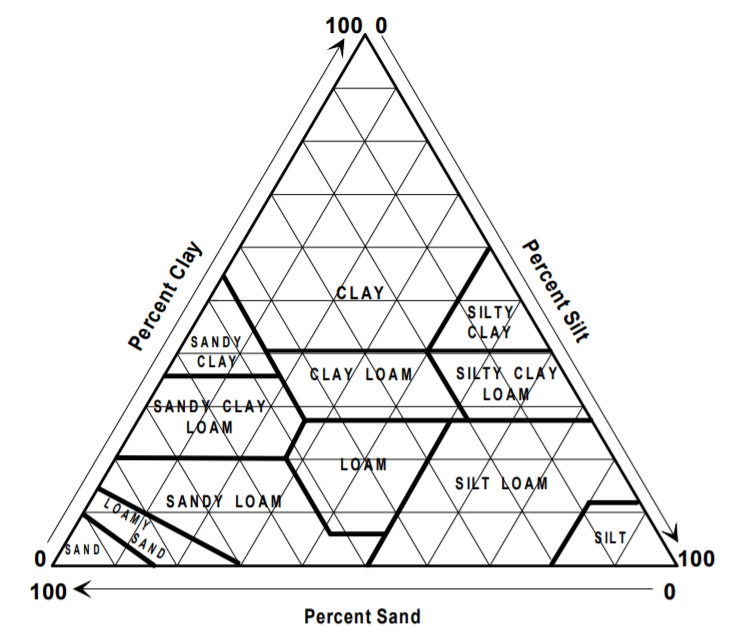
Soils are composed of particles with an infinite variety of sizes. The individual particles are divided by size into the categories of sand, silt and clay. Soil texture refers to the relative proportion of sand, silt and clay in the soil. Textural class is the name given to soil, based on the relative amounts of sand, silt and clay present, as indicated by the textural triangle shown in Figure 1.3. Such divisions are very meaningful in terms of relative plant growth. Many of the important chemical and physical reactions are associated with the surface of the particles, and hence are more active in fine than coarse-texture soils.
A textural class description of soils can tell a lot about soil-plant interactions,
since the physical and chemical properties of soils are determined largely by texture.
In mineral soils, exchange capacity (ability to hold plant nutrient elements) is related
closely to the amount and kind of clay in soils. Texture is a major determining factor
for water-holding capacity. Fine-textured soils (high percentage of silt and clay)
hold more water than coarse-textured soils (sandy). Water and air movement through
the finer-textured soils is reduced, making it more difficult to work.
Figure 1.3 Triangle for determining soil textural classes.
From the standpoint of plant growth, medium-textured soils, such as loams, sandy loams
and silt loams, are the most ideal. Nevertheless, the relationships between soil textural
class and soil productivity cannot be generally applied to all soils, since texture
is one of the many factors that influence crop production.
Check the texture of the surface and subsoil. Normally, the surface includes the top
foot of soil, but it may be shallower or deeper in certain situations. Soil below
the tillage zone is called subsoil. It is also necessary to consider the subsoil texture
when determining productivity potentials.
Soil Structure
Soil structure refers to the presence of aggregates of soil particles that have been bound together to form distinct shapes. Sometimes the binding or cementing is weak, however the aggregates are much larger than individual soil particles. Soil organic matter contributes significantly as a cementing agent. Air and water movement and root penetration in the soil is related to the soil structure. The better the structure, the higher the productivity of the soil.
Size and shape of the structure units is important. When height of the structure unit
is approximately equal to its width (blocky structure) we expect good air and water
movement. Structure units that have greater height than width (prismatic structure)
often are associated with subsoils that swell when wet and shrink when dry, resulting
in poor air and water movement. When particles have greater width than height (platy
structure), water and air movement and root development in the soil is restricted,
compared to a soil with desirable structure.
Granular structure, particularly in fine-textured soils, is ideal for water penetration
and air movement. Water and air move more freely through subsoils that have blocky
structure than those with platy structure. Good air and water movement is conducive
to plant root development. Types of soil structure are illustrated in Figure 1.4.
The productivity of the soil is influenced by both surface and subsoil texture and
structure. An approximate rating for soils considering texture and structure is shown
in Table 1.1.
Raise or lower the rating 10 to 20 percent, according to whether the soil structure
is more, or less, favorable than the average. If gravel occurs in the soil, lower
the rating according to its effect on the productive capacity.
Figure 1.4 Types of soil structure.
The productivity of the soil is influenced by both surface and subsoil texture and structure. An approximate rating for soils considering texture and structure is shown in Table 1.1. Raise or lower the rating 10 to 20 percent, according to whether the soil structure is more, or less, favorable than the average. If gravel occurs in the soil, lower the rating according to its effect on the productive capacity.
Table 1.1: Soil productivity rating as affected by texture.*
| Subsoil Texture |
Sand | Sandy Loam |
Loam | Clay Loam |
Clay; Silty Clay |
|---|---|---|---|---|---|
| ---Percent of Maximum Productivity--- | |||||
| Sandy | 50% | 55% | 65% | 60% | 55% |
| Sandy Loam | 60% | 70% | 80% | 75% | 65% |
| Loam | 70% | 80% | 95% | 90% | 75% |
| Clay Loam | 70% | 80% | 90% | 90% | 75% |
| Clay; Silty Clay | 65% | 70% | 80% | 80% | 70% |
* Numbers represent average soil conditions.
Soil Depth
Soil depth generally is used to describe how deep roots can favorably penetrate. Soils that are deep, well drained and have desirable texture and structure are suitable for production of most crops. For satisfactory production, most plants require considerable soil depth for root development to secure nutrients and water. Plants growing on shallow soils have little soil volume from which to secure water and nutrients. Depth of soil and its capacity to hold nutrients and water frequently determines crop yield, particularly for summer crops.
Roots of most crops extend 3 feet or more into favorable soil. Soils should be at least six feet deep to give maximum production. Look for materials or conditions that limit soil depth, such as hardpans, shale, coarse gravelly layers and tight impervious layers. These are almost impossible to change. A high water table may limit root growth, but it usually can be corrected by drainage. Soil productivity estimates on the basis of soil depth can be made using Table 1.2.
Table 1.2. Soil productivity rating as affected by depth.
| Soil Depth Usable by Crop (Feet) |
Roots Relative Productivity (Percent) |
|---|---|
| 1 | 35 |
| 2 | 60 |
| 3 | 75 |
| 4 | 85 |
| 5 | 95 |
| 6 | 100 |
Soil Slope
Topography of the land largely determines potential for runoff and erosion, method of irrigation and management practices needed to conserve soil and water. Higher-sloping land requires more management, labor and equipment expenditures.
Table 1.3 can be used to rate land productivity based on slope. If slope varies, use steeper slopes for the rating.
Erosion
Principal reasons for soil erosion in Oklahoma are 1) insufficient vegetative cover, which usually is a result of inadequate fertility to support a good plant cover, 2) growing cultivated crops on soils not suited to cultivation and 3) improper tillage of the soil. Soil erosion can be held to a minimum by 1) using the soil to produce crops for which it is suitable, 2) using adequate fertilizer and lime to promote vigorous plant growth and 3) using proven soil preparation and tillage methods.
Soils that have lost part or all their surfaces are usually harder to till and have lower productivity than non-eroded soils. To compensate for surface soil loss, more fertilization, liming and other management practices should be used.
Table 1.3. Soil productivity ratings as affected by slope.
| Slope ( % ) |
Relative Productivity Stable Soil ( % ) |
Relative Productivity Unstable, Easily Eroded soil ( % ) |
|---|---|---|
| 0-1 | 100 | 95 |
| 1-3 | 90 | 75 |
| 3-5 | 80 | 50 |
| 5-8 | 60 | 30 |
| 8-12 | 40 | 10 |
Soil and Available Water
Plants are totally dependent on water for growth and production. Even with well-fertilized soils, limited water can greatly reduce yields. Rainfall is not always dependable in Oklahoma. Therefore, crops are dependent on the moisture stored in the soil profile for growth and production.
Soils differ in their ability to supply water to plants. Limited root zones caused by shallow soils, high water table or claypans or extremely porous subsoils cause drought stress in plants faster than more desirable soils. Table 1.4 illustrates the differences in available water in selected soil profiles. Soils with silt loam or fine sandy loam surface textures have high available water-holding capacities. Differences in available water-holding capacity between the soils caused by widely varying textures of the subsoil and soil depth point out the need for knowing what is below the surface. (This kind of information is available in county soil survey manuals). During a drought, differences of 2 inches of available water can keep plants growing for an extra 10 days during peak plant use and could be the difference between success and crop failure.
Soil Fertility
Soil fertility is the soil’s ability to provide essential plant nutrients in adequate amounts and proper proportions to sustain plant growth. These nutrients and their functions are covered in details in the next chapter. Soil fertility is a component of soil productivity that is quite variable and strongly influenced by management. Other components of soil productivity, especially soil slope and soil depth, will be the same year after year. Together with climate, these components set the soil productivity limits, above which yields cannot be obtained even with ideal use of fertilizer. It is important to understand added fertilizer cannot compensate for an unproductive soil due to it being excessively stony or has a subsoil layer that restricts normal root growth and development. This point is illustrated in Figure 1.5.
Table 1.4. Effect of depth and texture on available water for crop use.
| Soil Name | Texture | Depth (inches) |
Available Water (inches) |
|---|---|---|---|
| Dennis | Silt loam Silty clay loam Clay |
0-11 11-23 23-60 |
1.98 2.52 5.55 |
| TOTAL | 60 | 10.05 | |
| Sallisaw | Silt loam Silt loam Gravelly clay loam Very gravelly clay loam |
0-10 10-20 20-40 40-60 |
1.8 1.8 2.8 1.6 |
| TOTAL | 60 | 8 | |
| Shellabarger | Fine sandy loam Sandy clay loam Fine sandy loam |
0-16 16-52 52-60 |
1.92 5.86 0.88 |
| TOTAL | 60 | 8.66 | |
| Stephenville | Fine sandy loam Sandy clay loam Sandstone |
0-14 14-38 38+ |
1.82 3.84 - |
| TOTAL | 38+ | 5.66 |
Figure 1.5: Influence of soil productivity on yield response to fertility.
Soil Management
There are numerous other soil characteristics that can be important to soil productivity in specific areas. These include: soil drainage, soli salinity, presence of stone and/or rocks and organic matter content. They are not major limiting factors over wide areas and will not be discussed here.
One additional factor on which soil productivity is highly dependent is soil management. This implies using the best available knowledge, techniques, materials and equipment in crop production. The use of minimum tillage is an important management practice used to reduce the potential damage to soil structure from overworking, and for economic and fuel conservation purposes, as well as to allow farming of more acres per unit of labor.
Soil conservation is a concept integrating important management practices that deserves close attention. In the U.S., it is estimated that four billion tons of sediment are lost annually from the land in runoff waters, and with it much of the natural and applied fertility. That is equivalent to the total loss of topsoil (6 inches deep) from four million acres. Wind erosion is also a problem in certain areas. Management practices such as contouring, strip planting, cover cropping, reduced tillage, terracing and crop residue management help eliminate or minimize the loss of soil from water and wind erosion.
Proper utilization of crop residues can be a key management practice. Crop residues returned to the soil improve soil productivity through the addition of organic matter and plant nutrients. The organic matter also contributes to an improved physical condition of the soil, which increases water infiltration and storage and aids aeration. This is vital to crop growth.
Summary
Limitations of soil, water or climate reduce the soil’s ability to produce. These limitations increase the need for better management practices. Poor management, or the presence of weeds, compact soils, soil erosion, etc., will result in low yields even on the most productive soils. On the other hand, good management on moderately productive soils can give high yields. By considering the factors discussed in this chapter, one can make a better determination of the soil’s overall crop productivity and make better decisions about nutrient management including use of fertilizers.
Chapter 2 - Essential Plant Nutrient Functions, Soil Reactions and Availability
Bill Raun
More than 100 chemical elements are known to man today. However, only 16 have been proven to be essential for plant growth. For a nutrient to be classified as essential, certain rigid criteria must be met. The criteria are as follows:
- The element is essential if a deficiency prevents the plant from completing its vegetative or reproductive cycle.
- The element is essential if the deficiency in question can be prevented or corrected only by supplying the element.
- The element is essential if it is directly involved in the nutrition of the plant and is not a result of correcting some microbiological or chemical condition in the soil or culture media.
The essential elements and their chemical symbols are listed in Table 2.1. Three of the 16 essential elements – carbon, hydrogen and oxygen – are supplied mostly by air and water. These elements are used in relatively large amounts by plants and are considered to be non-mineral, since they are supplied to plants by carbon dioxide and water. The non-mineral elements are not considered fertilizer elements. The other 13 essential elements are mineral elements and must be supplied by the soil and/or fertilizers.
Table 2.1. Essential plant nutrients, chemical symbols and sources.
| Mostly from air and water (non-mineral) | Mostly from air and water (non-mineral) | From soil and/or fertilizers (mineral) | From soil and/or fertilizers (mineral) | From soil and/or fertilizers (mineral) | From soil and/or fertilizers (mineral) |
|---|---|---|---|---|---|
| Element | Symbol | Element | Symbol | Element | Symbol |
|
Carbon |
C |
Nitrogen |
N |
Iron |
Fe |
|
Hydrogen |
H |
Phosphorus |
P |
Manganese |
Mn |
|
Oxygen |
O |
Potassium |
K |
Zink |
Zn |
|
|
|
Calcium |
Ca |
Copper |
Cu |
|
|
|
Magnesium Mg |
Mg |
Boron |
B |
|
Sulfur |
S |
Molybdenium |
Mo |
||
|
Chlorine |
Cl |
The essential plant nutrients may be grouped into three categories. They are as follows:
- Primary nutrients - nitrogen, phosphorus and potassium
- Secondary nutrients - calcium, magnesium and sulfur
- Micronutrients - iron, manganese, zinc, copper, boron, molybdenum and chlorine
This grouping separates the elements based on relative amounts required for plant growth, and is not meant to imply any element is more essential than another.
Primary Non-Mineral Nutrients
Carbon, Hydrogen and Oxygen
Carbon is the backbone of all organic molecules in the plant and is the basic building block for growth. After absorption of carbon dioxide (CO2) by the leaves of the plant, carbon is transformed into carbohydrates by combining with hydrogen and oxygen through the process of photosynthesis.
Metabolic processes within the plant transform carbohydrates into amino acids and proteins and other essential components.
Primary Mineral Nutrients
Nitrogen
Nitrogen is an integral component of amino acids, which are the building blocks for proteins. Proteins are present in the plant as enzymes that are responsible for metabolic reactions in the plant. Because nitrogen is so important, plants often respond dramatically to available nitrogen.
Soil Nitrogen Reactions and Availability
Most of the nitrogen in Oklahoma soil is present as organic nitrogen in the soil organic matter. There are about 1,000 pounds per acre of nitrogen in this form for every one percent organic matter in the soil. However, since the soil organic matter is resistant to further decay, most of this nitrogen is unavailable during any given growing season. Normally, about two percent of the nitrogen from soil organic matter will be released each year to mineral forms when soils are cultivated. This 20 to 40 pounds per acre of nitrogen is typical of the amount present in unfertilized soils after cultivation and seed bed preparation.
Nitrogen Mineralization and Immobilization
Because nitrogen release from organic matter is dependent upon decay by microorganisms, which themselves require nitrogen, the amount of nitrogen available for a crop is in constant flux. Unlike crops, which get their carbon as carbon dioxide from the air, many microorganisms get their carbon by decaying organic matter. Nitrogen availability depends upon the relative amount of carbon and nitrogen in the organic matter, its resistance to decay, and environmental conditions to support microbial activity. Figure 2.1 illustrates how nitrogen becomes more concentrated as soil organic matter decays.
Figure 2.1: Narrowing of carbon to nitrogen ratio as residue decay until mineral nitrogen finally becomes available.
Note that nitrogen is not released during the first stages of decay. This is because nitrogen that is released is immediately consumed by active microorganisms. With time, remaining organic material becomes more resistant to decay, microorganisms die off, and there is more mineral nitrogen present than can be consumed by the few active microorganisms. This results in a final release of measurable mineral nitrogen in the form of ammonia (NH3). The ammonia readily reacts with soil moisture to form ammonium (NH4+). These two reactions can be stated simply as:
organic nitrogen → NH3 (gas) [1]
NH3 + H2 O → NH4+ + OH- [2]
ammonia + water ammonium + hydroxide
The process of converting or transforming nitrogen from organic compounds to inorganic compounds is called mineralization. This results in increasing nitrogen available for crops. When the reverse happens, and available nitrogen is absorbed by crops or microorganisms, the process is called immobilization and results in a decrease in the amount of nitrogen immediately available for crops. These processes and their interacting nature with soil nitrogen for a typical field situation are illustrated in Figure 2.2.
Approximately 98 percent of the soil nitrogen is unavailable for plant uptake. This large reservoir of organic nitrogen provides an important buffer against rapid changes in available nitrogen and plant stress. The small reservoir of mineral nitrogen can often be slowly replenished by mineralization (Figure 2.2) when crops need additional nitrogen.
Figure 2.2: Interacting pools of soil nitrogen.
Supplemental nitrogen as fertilizer usually must be added to support high, economic production levels. Immediately following fertilization with 120 pounds nitrogen, the system may be illustrated by Figure 2.3a. Addition of fertilizer nitrogen will stimulate microorganism activity, resulting in consumption of nitrogen and breakdown of some crop residues (immobilization) as illustrated in Figure 2.3b. The immobilized nitrogen will be present as microbial tissue and other new material in the organic pool. As indicated by the two arrows pointing in opposite pathways, mineralization and immobilization are both taking place simultaneously. Immobilized fertilizer nitrogen will again become available in a few weeks if conditions favor crop uptake.
Figure 2.3: Relative amounts of organic and mineral nitrogen in soil immediately after fertilizing (a) and several days after active immobilization (b).
Nitrification
In addition to the general mineralization and immobilization reactions, other reactions also are responsible for nitrogen changes (transformations) in the soil. Nitrification is one of the first reactions to occur after organic nitrogen has been converted to ammonium-N. This change is also a result of aerobic microorganism activity as depicted in the following reaction.
2NH4+ + 3O2 → 2NO2- + 2H2O + 4H+ [3]
ammonium oxygen nitrite water hydrogen ion
This reaction produces nitrite-N and hydrogen ions. Since hydrogen ions are generated, it is easy to see this step will at least temporarily contribute to soil acidity. However, this production of acidity is partially compensated for by the hydroxide (OH-) produced from reaction [2]. The hydrogen and hydroxide will combine to form water, so the net effect on acidity when organic nitrogen is mineralized will be 1 pound of hydrogen produced for every 14 pounds of nitrogen mineralized. The same reactions and acidity will occur when fertilizer nitrogen is added in the ammonia form (anhydrous ammonia or urea). Ammonium sulfate will be twice as acidifying because equation [2] will be avoided by adding the ammonium (NH4+) form of nitrogen.
Almost immediately after nitrite (NO2-) nitrogen is produced (reaction [3]), a companion reaction occurs that is also carried out by soil microorganisms resulting in nitrate-N (NO3 - N) being produced from nitrite.
2NO2- + O2 → 2NO3- [4]
Because this change is quite rapid compared to the change from ammonium to nitrite [3] there is seldom any nitrite (NO2-) present in soils. Ammonium and nitrate are common and will increase or decrease depending on microbial activity that will both generate and consume ammonium and nitrate. This cyclic interaction of nitrogen transformations is shown in Figure 2.4.
Whenever nitrate and/or ammonium nitrogen are measured in the soil, these measurements provide a view of two components of the nitrogen cycle at a single point in time. If the measurement is made when the system is likely to be in balance, or equilibrium, such as when wheatland soils are tested for nitrate in July or August, the value can be a useful guide for determining nitrogen fertilizer needs. Figure 2.5 illustrates the changes that took place for ammonium and nitrate nitrogen in soil during wheat production under different rates of fertilizer use. Because ammonium and nitrate nitrogen are the two forms of nitrogen that higher plants utilize, these two forms have received the greatest attention.
Figure 2.4: Primary forms of nitrogen in soils and the transformations among them. (1) Decay of soil organic matter releasing ammonia; (2) reaction of ammonia with water to form ammonium; (3) transformation of ammonium to nitrate by microorganisms; (4) uptake of ammonium and/ or nitrate by plants and microorganisms; (5) plants eaten by animals; (6) animal manures, nitrogen fixation and plant residue return to soil; (7) residues decay to resistant organic matter, ammonia produced from nitrogen rich materials; (8) soil organic matter produced as decay continues.
Soil fertility research at OSU has documented the change of ammonium and nitrate nitrogen following fertilization (Figure 2.5). Only about 60 percent of the fertilizer nitrogen could be accounted for at the first sampling after fertilization. This was mostly present as nitrate although the fertilizer (ammonium nitrate) was an equal mixture of the two nitrogen forms measured. In the short period after application, some transformations had taken place. These continued, resulting in a gradual increase in ammonium nitrogen (probably from some mineralization) and a rapid decline in nitrate, likely from immobilization caused by microbial activity and uptake by the wheat crop.
Figure 2.5. Surface soil (0-6”) ammonium and nitrate nitrogen following fertilization at different rates from OSU Soil Fertility Research.
When crop production is added to the cycle in Figure 2.4, it becomes obvious that the cycle is not self sustaining. Harvesting removes significant amounts of nitrogen each year and eventually the system becomes depleted in organic matter and available nitrogen to support normal crop yields. A common response is to add nitrogen back using legumes and commercial fertilizers. When additions are balanced with removals, soil organic matter and productivity can potentially be sustained. However, excessive tillage, residue removal (straw and chaff in wheat production) and residue burning often result in continued soil organic matter decline. This loss in soil organic matter can lead to more pronounced surface crusting following rain.
Nitrogen Fixation
Additions to soil nitrogen are made as a result of either atmospheric, biological or industrial fixation of atmospheric nitrogen (N2). These processes are responsible for transforming nitrogen from the atmosphere to either ammonium or nitrate nitrogen that can be used by plants. The atmosphere contains an inexhaustible amount (78 percent) of nitrogen. Approximately 35,000 tons of nitrogen are present in the atmosphere above each acre of the earth’s surface.
Atmospheric nitrogen fixation occurs when there is electrical discharge or lightning
during thunderstorms. This causes elemental nitrogen (N2) to combine with elemental oxygen (O2) to form nitrate (NO3-). The nitrate is added to the soil with rainwater and accounts for about 3 to 5
pounds of nitrogen per acre per year.
Biological nitrogen fixation can be either symbiotic or non-symbiotic. Symbiotic nitrogen
fixation occurs within legumes. Bacteria (rhizobium sp.) infect the root of the legume
and cause a nodule to form. The rhizobium obtain their energy from the legume and
convert free nitrogen to ammonia (NH3), which the host plant utilizes to make amino acids and proteins. Legumes may fix
as much as 500 pounds of nitrogen per acre per year (alfalfa) by this process. However,
only a small fraction of the nitrogen fixed by legumes will be available for subsequent
crops unless the legume is “plowed down” when a significant amount of top growth is
present. Normally, most of the fixed nitrogen is removed in the harvest. Typical amounts
of nitrogen added from legumes are shown in Table 2.2.
Biological nitrogen fixation is an extremely important source of adding nitrogen to
soils when fertilizer nitrogen is unavailable. In Oklahoma, the addition of nitrogen
to soils as a result of growing legumes is significant and should always be accounted
for when determining nitrogen needs for non-legume crops in the subsequent season.
However, the cost of establishing and growing legumes for this purpose alone, precludes
their use as a sole substitute for nitrogen fertilizers.
Non-symbiotic nitrogen fixation is accomplished by certain “free-living” microorganisms
(cyanobacteria or blue-green algae), which live independently of other living tissue.
The total contribution of nitrogen from these microorganisms can actually be significant.
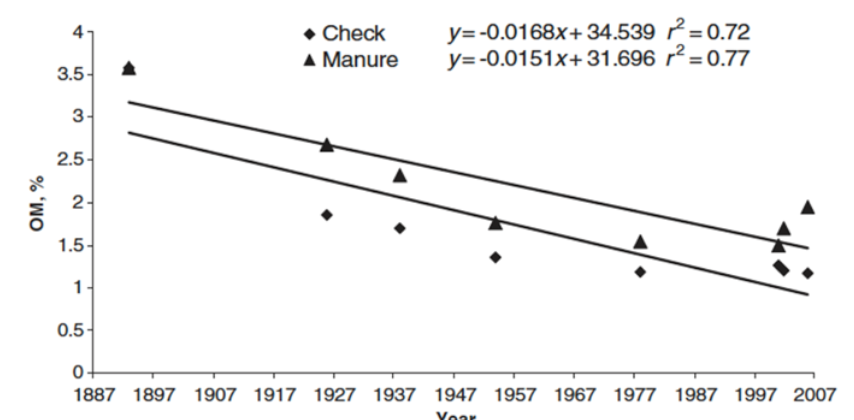
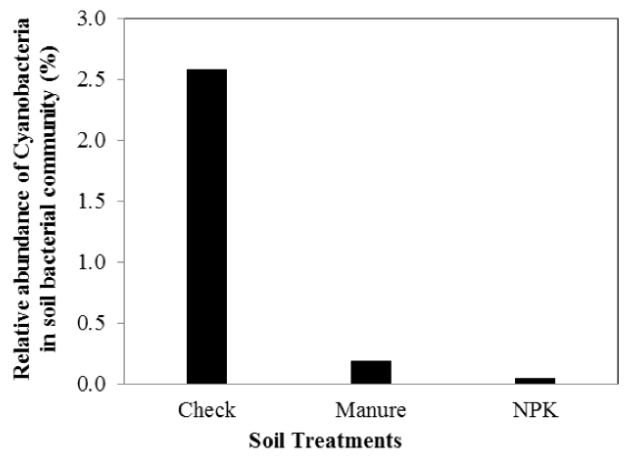
Recent studies from the Magruder Plots started in 1892 found cyanobacteria in the
check plot where no nitrogen has ever been applied. This helps to explain why wheat
yields in these plots continue to be around 20 bushels per acre, more than 120 years
later with no nitrogen additions.
Table 2.2 Average nitrogen remaining (N-credit) in the soil after legume crops.
| Legume | N-credit (lb N/acre) | Legume | N-credit (lb N/acre) |
|---|---|---|---|
| Alfalfa | 80 | Cowpeas | 30 |
| Ladino clover | 60 | Lespedeza (annual) | 20 |
| Sweet clover | 60 | Vetch | 40 |
| Red clover | 40 | Peas | 40 |
| Kudzu | 40 | Winter peas | 40 |
| White clover | 20 | Peanuts | 20 |
| Soybeans | 20 | Beans | 20 |
Industrial fixation of nitrogen involves reacting atmospheric nitrogen (N2) with hydrogen (H), usually in the form of natural gas, under high temperature and pressure to form ammonia (NH3 ). The ammonia may be used directly as anhydrous ammonia gas or converted to other nitrogen fertilizers such as urea, ammonium nitrate, urea-ammonium nitrate solution, ammonium sulfate or ammonium phosphates. Industrial fixation in Oklahoma is responsible for additions of about 300,000 tons of nitrogen per year. This amount of nitrogen is roughly equal to nitrogen removed in harvested crops.
Nitrogen fixation results in addition of nitrogen to the soil through utilization by plants and their residues subsequently added back to the soil (Figure 2.6). In order for soil organic matter to be maintained it is necessary for these additions to be at least equal to the amount of nitrogen removed from the field by harvest. Figure 2.6 illustrates how nitrogen fixation interacts with other forms of nitrogen and their transformations.
Figure 2.6: Addition of nitrogen to the nitrogen cycle from fixation of atmospheric nitrogen by: (9) lightning; (10) symbiosis with legumes; (11) industrial fertilizer plants.
Nitrogen Losses
The major nitrogen loss from soils is the removal of nitrogen by harvest of non-legume crops. Other significant nitrogen losses include:
- Volatilization of ammonia.
- Volatilization of nitrous oxide (N2O) and nitric oxide (NO) from nitrate in poorly aerated soils (denitrification).
- Leaching of nitrate out of the root zone in permeable soils receiving heavy rainfall or irrigation.
- Plant nitrogen loss as ammonia from plants containing nitrogen in excess of what the plant can use in seed production, just after flowering.
Each of these processes is responsible only for very small amounts of nitrogen loss over the course of a crop growing season. However, when considered over a generation of farming, or even just a few years, the amount of nitrogen lost can be significant. Nitrogen losses by these processes are responsible for the fact only 30 to 40 percent of fertilizer nitrogen applied can be found in the crop at harvest. Research at OSU and other institutions continues to examine practices that will improve fertilizer-nitrogen-use efficiency. Figure 2.7 illustrates the interaction of these nitrogen losses with other forms of nitrogen and their transformations.
Phosphorus
Most of the total phosphorus in soils is tied up chemically in compounds with low solubility. In neutral- to alkaline-pH soils, calcium phosphates are formed, while in acid soils, iron and aluminum phosphates are produced.
Soil Phosphorus Reactions and Availability
Available soil phosphorus, or that fraction which the plant can use, makes up about
one percent or less of the total phosphorus in soils. The availability of inorganic
phosphorus in soils is related to the solubility of specific phosphorus compounds
present. Phosphorus solubility in particular is controlled by a number of factors
– most importantly soil pH.
The amount of precipitated phosphorus is one factor. The greater the total amount
present in soil, the greater the chance to have more phosphorus in solution. Another
important factor is the extent of contact between precipitated phosphorus forms and
the soil solution. Greater exposure of phosphate to soil solution and plant roots
increases its ability to maintain replacement supplies. During periods of rapid growth,
phosphorus in the soil solution may be replaced 10 times or more per day from the
precipitated or solid phase. The rate of dissolution and diffusion of soluble phosphorus
determines soil phosphate availability. As phosphate ions (mainly H2PO4- and HPO42-) are taken up by the plant, more must come from the solid phase.
Soil pH can be a controlling factor that determines phosphorus solubility. Maximum
phosphorus availability occurs in a pH range of 5.5 to 7.2. At soil pH levels below
5.5, iron (Fe), aluminum (Al) and manganese (Mn) react with phosphorus to form insoluble
compounds. When soil pH exceeds 7.2, phosphorus will complex with calcium (Ca) to
form plant-unavailable phosphorus forms. However, it should be noted the solubility
of calcium phosphates is much greater than aluminum and iron phosphates.
Figure 2.7: Losses of nitrogen from the nitrogen cycle as a result of: (12) ammonia volatilization; (13) transformation of nitrate to gaseous oxides (denitrification); (14) leaching below the root zone; (15) volatilization from crops; and (16) harvest removal.
The proportion of total soil phosphorus relatively available is dependent upon time of reaction, type of clay present in the soil, organic matter content and temperature. The solubility of phosphate compounds formed from added phosphorus due to time of reaction can be broken down in three major groups (Figure 2.8). Fertilizer phosphates are generally in the readily available phosphate group but are quickly converted to slowly available forms. These can be utilized by plants at first, but upon aging are rendered less available and are then classified as being very slowly available. At any one time, 80 to 90 percent of the soil phosphorus is in very slowly available forms. Most of the remainder is in the slowly available form since less than 1 percent would be expected to be readily available.
The formation of insoluble phosphorus containing compounds in soils that renders phosphorus unavailable for plant use is called phosphorus fixation. Each soil has an inherent fixation capacity that must be satisfied in order to build available phosphorus levels. In Oklahoma, a large portion of the clays have a lower fixation capacity than the highly weathered soils found in high rainfall areas. It is important to understand the actual amount of phosphorus in the soil and the amount available to crops will not necessarily be reflected in a soil test. These soil tests simply provide an index of sufficiency and not an index of build-up or accumulation. Because different soils will have differing fixation capacities, the importance of annual soil testing becomes clear, since this practice is the only method used to estimate future crop fertilizer needs. In addition, these tests should reflect past management (farmers applying more than enough or not enough on an annual basis), and farmers thus can compensate accordingly.
Very slowly available phosphates
Apatites, aged Fe, Mn and Al phosphates,
stable organic phosphates
⇑ ⇓
Slowly available phosphates
Ca3 (PO4)2 , freshly formed Fe, Al, Mn phosphates
(small crystals) and mineralized organic phosphates
⇑ ⇓
Readily available phosphates
Water-soluble
ammonium phosphates
NH4 H2 PO4 (MAP 11-52-0)
(NH4 )2 HPO4 (DAP 18-46-0)
monocalcium phosphate
Ca(H2 PO4 )2 (0-46-0)
Water-insoluble
dicalcium phosphate
CaHPO4
Figure 2.8: Relative availability of different phosphate forms and their transformations.
Organic matter and microbial activity affect available soil phosphorus levels. As was the case with nitrogen, the rapid decomposition of organic matter and consequent high microbial population results in temporary tying up of inorganic phosphorus (immobilization) in microbial tissue, which later is rendered available through release (mineralization) processes. This is one of the reasons why broadcasting phosphorus in zero/minimum tillage systems can be beneficial, especially where soil phosphorus fixation capacities are high.
Less than 30 percent of phosphorus fertilizers applied is recovered in plants. Therefore, due to fixation reactions, more phosphorus must be added than is actually removed by crops. Legumes, in general, require much larger amounts of phosphorus than many of the common grain crops grown in Oklahoma.
Because phosphorus is immobile in the soil, roots must come in direct contact with this element before the plant can take it up. However, phosphorus is mobile within the plant and if deficient, lower leaves generally will demonstrate purple coloration on the outer edge of the leaf and/or the leaf margins.
Over a wide range of soils and cropping conditions, phosphorus has proven to be one of the more deficient elements in Oklahoma production agriculture. Soil testing on an annual basis should assist in determining crop needs.
Potassium
Plants take up potassium as the potassium ion (K+). Potassium within plants is not synthesized into compounds and tends to remain in ionic form in cells and plant tissue. Potassium is essential for photosynthesis, starch formation and translocation of sugars within plants. It is necessary for the development of chlorophyll, although it is not part of its molecular structure.
The main functions of potassium in plants are in the translocation of sugars and its involvement in photosynthesis.
Soil Potassium Reactions and Availability
In most soils (except extremely sandy soils in high rainfall regions), total potassium contents are high. Similar to nitrogen and phosphorus, not all of the total potassium is available for plant growth. The relationship of unavailable, slowly available and readily available forms of potassium is illustrated in Figure 2.9. Only 1 to 2 percent of the total potassium in soils is readily available. Of this, approximately 90 percent is exchangeable or attached to the outside edge of clays, and the remaining 10 percent is in the soil solution. Equilibrium exists between the nonexchangeable, exchangeable and water soluble forms. When the plant removes potassium from the water soluble form, the concentration is readjusted by the exchangeable and nonexchangeable forms. In the case of added potassium, some of the available forms will move toward nonexchangeable forms. The nonexchangeable form also may be referred to as fixed. Certain 2:1 type clay minerals have pore space large enough for the potassium ions (K+) to become trapped, rendering the ions unavailable for immediate plant use. Potassium is positively charged and clays are negatively charged and this makes the potassium ion relatively immobile in the soil. Except in extremely sandy soils, leaching losses under normal Oklahoma conditions are minimal. The largest loss comes from crop removal, particularly where hay crops are produced. Most of western Oklahoma soils have adequate plant available potassium, however, this can best be determined for individual fields by soil testing.
Relatively Unavailable Potassium (Feldspars, Micas, etc.) 90 to 98% of total potassium
⇒ Slowly Available Potassium (Nonexchangeable (fixed)) 1 to 10% of total potassium
⇑ ⇓
⇒ Readily Available Potassium (Exchangeable and solution) 1 to 2% of total potassium
Figure 2.9: Relative amounts of soil potassium present in different levels of availability to plan
Secondary Mineral Elements
Nutrients that are used in relatively moderate amounts by most plants have been categorized as secondary nutrients. These nutrients are calcium (Ca), magnesium (Mg) and sulfur (S).
Calcium
Calcium is taken up by plants as the cation, Ca2+. Calcium functions in the plant in cell wall development and formation. Calcium is not translocated in plants and consequently, the deficiency of calcium will be observed first in the new, developing plant tissue. Calcium deficient tissue fails to develop normal morphological features and will appear to be an undifferentiated gelatinous mass in the region of new leaf development.
The calcium ion is referred to as a basic ion because the element reacts with water to form the strong base calcium hydroxide, Ca(OH)2 . Calcium is held tightly on the negatively charged clay and organic particles in soils and is abundant in soils that have developed in arid and semi-arid climates. Because of this, it is primarily responsible for maintaining these soils at or near a neutral pH. In addition to unweathered primary and secondary minerals, soils often contain calcium in the form of impure lime (calcium carbonate, CaCO3 ) and gypsum (calcium sulfate, CaSO4 ). Except in the production of peanuts on sandy, acid soils, calcium deficiency in Oklahoma crops has not been substantiated by research. However, because calcium absorption by the developing peanut pod is not very effective from soils with a marginal supply of calcium, peanut producers often apply gypsum over the pegging zone just before the plant begins to peg to assure the crop will be adequately supplied with calcium. For most soils, before the available calcium level reaches a critically low point, the soil pH will become so low that soil acidity will be a major limitation to crop production. Since the common correction of acid soils is to add lime in amounts of tons per acre, this practice will incidentally maintain a high level of available calcium for crops.
Magnesium
Magnesium is absorbed as the divalent cation, Mg2+, and functions in many enzymatic reactions as a co-factor or in a co-enzyme. The most noteworthy function of magnesium in plants is as the central cation in the chlorophyll molecule. Without magnesium, plants cannot produce adequate chlorophyll and will lose their green color and ability to carry out photosynthesis, the process responsible for capturing energy from sunlight and converting it into chemical energy within the plant. Magnesium deficiency will result in yellow, stunted plants.
Magnesium reactions in soils are similar to calcium in many respects. Magnesium, like calcium, is a basic ion that generally is abundant in arid and semi-arid soils with near neutral pH. Deficiencies most often occur in deep sandy soils with a history of high forage production (8 to 10 tons per acre annually), where forage has been removed as hay. In Oklahoma, deficiencies have occasionally been noted under these conditions in the eastern half of the state. Like calcium, deficiencies are likely to occur on acid soils, and since most lime will contain a small amount (2 to 5 percent) of magnesium carbonate, liming acid soils on a regular basis usually will assure an abundant supply of plant available magnesium. If magnesium deficiency is a reoccurring problem, dolomitic lime (primarily magnesium carbonate) should be sought as a liming source.
Sulfur
Sulfur is absorbed by plants as the sulfate anion, SO42- . Sulfur is a component of three of the 21 essential amino acids and thus, is critical to the formation and function of proteins. Sulfur deficiency causes plants to become light green and stunted. Most crops require about 1/20 the amount of sulfur that they do of nitrogen. Bumper yields of most crops can be supported by 5 to 15 pounds per acre of sulfur.
Sulfur is found in soil in the form of soil organic matter (like nitrogen), dissolved in the soil solution as the sulfate ion and as a part of the solid mineral matter of soils. Sulfur compounds, such as gypsum, are slightly soluble in water. Like nitrate nitrogen, the negatively charged sulfate ion is not readily adsorbed to clay and humus particles and may be leached into the subsoil with a porous surface soil layer. Sulfur deficiencies most often occur in deep sandy soils, low in organic matter, with a history of high crop production and removal. Soils that have a well developed B horizon seldom will be deficient in sulfur because sulfur will not leach out of the root zone and the accumulated sulfur in the subsoil will adequately satisfy crop needs. This is one of the reasons why early sulpher deficiencies often disappear at late stages of growth, at which time roots have penetrated subsoil horizons rich in sulfur. Plant deficiencies in general show up on the younger leaves, with light yellow discoloration. Soils that contain normal amounts of organic matter will release sulfur by mineralization, much like nitrogen, and this will contribute significantly to meeting crop needs. Sulfur deficiencies in Oklahoma are very rare because on the average there is about 6 pounds per acre of sulfur added to soils annually in the form of rainfall. Sulfur is still added incidentally as a component of phosphate fertilizers and other agricultural chemicals which contribute significantly to the requirement of crops. Also, Oklahoma irrigation waters are usually high in sulfate, and add significant amounts each year (for every ppm of sulfate-S, 2.7 pounds per acre of sulfur is added for each acre-foot of irrigation).
Micronutrients
The micronutrients are grouped together because they are all required by plants in very small amounts. Some, like molybdenum (Mo), are required in such small amounts that deficiencies can be corrected by applying the element at only a fraction of a pound per acre. Similarly, chlorine is needed in such small quantities that when researchers at the University of California were attempting to document its necessity, they found that touching plant leaves with their fingers transferred enough chlorine from the perspiration on their skin to meet the plant’s requirements. These elements do not function in plants as a component of structural tissues like primary and secondary nutrients. Instead, micronutrients are mainly involved in metabolic reactions as a part of enzymes where they are used over and over without being consumed. Nevertheless, their functions are very specific and cannot be substituted for by some other element. Deficiencies of any of the elements can be corrected by foliar application of solutions containing the element.
Manganese, Chlorine, Copper and Molybdenum
Deficiencies of these nutrients have yet to be documented in Oklahoma, except for chlorine in wheat on a deep sandy soil near Perkins. Each of the elements is adsorbed by plants in the ionic form, manganese and copper as the divalent cations Mn2 + and Cu2 +, molybdenum as the oxyanion MoO42 -, and chlorine as the simple Cl- anion. Of these four nutrients, molybdenum and chlorine are probably the most likely to receive attention. Molybdenum functions in plants in the enzyme nitrate reductase, which is very important in nitrogen metabolism in legumes. Availability is reduced in acid soils and often if molybdenum availability is marginal it can be increased to adequate levels by simply liming the soil. Where large seeded legumes are grown, like soybeans or peanuts, obtaining seed that was grown with a good supply of molybdenum will avoid the deficiency because normal levels of molybdenum in the seed will be enough to meet the plant needs.
Soil fertility research in the Great Plains has occasionally shown small grain response to fertilizers containing chlorine. Often the response has been the result of disease suppression (take-all disease) rather than correction of an actual nutrient deficiency in the plant, and usually it has been in areas that do not commonly apply potassium fertilizers containing chloride (such as muriate of potash or potassium chloride, 0-0-62).
Boron
Boron is absorbed by plants as uncharged boric acid, B(OH)3 , the chemical form also present in soil solution. Boron is believed to function in plants in the translocation of sugars. Because B is uncharged in soil solution and it forms slightly soluble compounds, it also is relatively mobile in soils and can be leached out of the surface soil. This is sometimes critical in peanut production because of the very sandy, porous soils peanuts are produced in. As a result, boron deficiency has been reported in peanuts. The deficiency manifests itself as a condition known as “hollow heart” whereby the center of the nut is not completely filled and an inferior crop is harvested. Although alfalfa has an annual requirement twice that of peanuts, the deficiency of boron has never been documented in alfalfa. The reason for this is likely because alfalfa is usually grown in deep, medium textured soils and because alfalfa has an extensive root system even at lower depths in the soil profile. Whenever boron deficiencies are suspected, and if boron fertilizer is applied, care should be exercised as toxicities can be created by simply doubling the recommended rate.
Iron and Zinc
Iron and zinc deficiencies both occur in Oklahoma and are associated with unique soil and crop situations. Zinc is absorbed as the divalent cation Zn2+, while iron is absorbed as a “plant provided” chelated Fe3+ complex by grass type plants and as the “plant-reduced” divalent cation Fe2+ by broad-leaved plants.
Corn is sensitive to moderately low soil zinc levels and deficiencies may occur at DTPA soil test values below 0.8 parts per million. Winter wheat, on the other hand, has been grown in research experiments near Woodward, Oklahoma where the soil test zinc value was less than 0.15 parts per million without showing any deficiency or responding to zinc fertilizer. Obviously winter wheat is very effective in utilizing small amounts of soil zinc. Zinc deficiencies in corn are most common where fields have been leveled or for some other reason the topsoil has been removed and the surface soil has very little organic matter and where the subsoil pH is high. Deficiencies are easily corrected by broadcast application of about 4 to 6 pounds per acre of zinc preplant. An application of this rate should remove the deficiency for 3 to 4 years. The most sensitive plant to zinc deficiency in Oklahoma is pecans. Deficiencies may occur whenever DTPA soil test values are less than 2.0 parts per million. Foliar sprays are very effective in preventing and/or correcting the deficiency. A single application usually lasting the entire growing season.
Iron deficiency is most common in sorghum and sorghum-sudan crops in Oklahoma. The occurrence is limited to the western half of the state in soils that are slightly alkaline (pH above 7.5). All soils in Oklahoma contain large amounts of iron, usually in excess of 50,000 pounds per acre. However, almost all of this iron is in a form that is not available to crops, like rust. Iron availability is increased greatly in acid soils, consequently the deficiency is seldom observed in any crops in eastern and central Oklahoma, where soil pH is usually less than 7.0. Iron deficiency cannot be corrected by soil application of iron-containing fertilizers because the iron from the fertilizer is quickly converted to unavailable iron just like that already present in the soil. The exception to this general rule is the use of chelated iron. However, these fertilizer materials can be cost prohibitive for field scale use. Foliar application of iron sulfate solutions is effective for supplying iron to deficient plants. Unfortunately, iron is not translocated in the plant and subsequent new leaves will again exhibit the interveinal chlorosis (yellow between the veins) characteristic of iron deficiency. Repeated spraying will provide iron for normal growth but often will be cost prohibitive. The most effective long-term corrective measure for dealing with iron chlorosis is to increase soil organic matter since iron deficiency usually is limited to small areas of a field. Organic matter can be effectively increased by annual additions of animal manure or rotted hay. This results in additional chelating of iron and also has a tendency to acidify the soil. Broadleaf plants have what is called an “adaptive response mechanism” that allows them to make iron more available if they experience iron stress. The strength of this mechanism is a genetic trait and some varieties, such as ‘forest’ soybeans, do not possess this ability and will often become chlorotic if grown in neutral or alkaline soils.
The Mobility Concept
The nutrient mobility concept as it relates to soil fertility was first proposed in 1954 by Roger H. Bray at the University of Illinois. Much research since then has supported his mobility concept and it is now considered basic to the understanding of soil fertility. Bray simplified all the soil chemistry surrounding the essential nutrients to the fact that some are quite mobile in soils and others are relatively immobile.
Mobile Nutrients
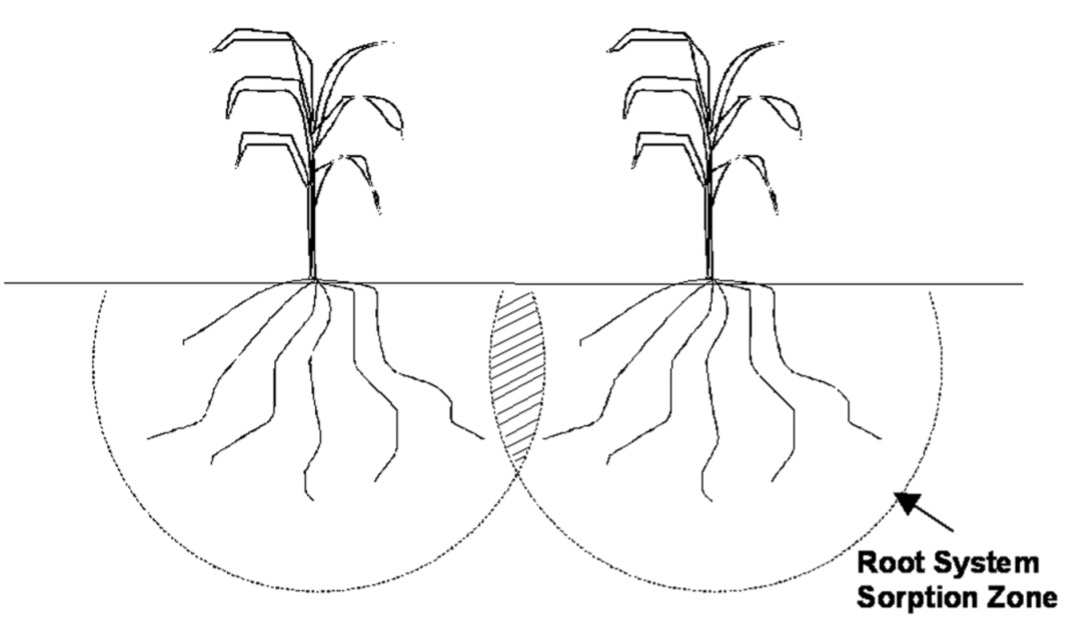
Plants are able to extract mobile nutrients from a large volume of soil, even soil beyond the furthest extension of their roots because as the plants extract water from around their roots, water from further away moves toward the root and carries the mobile nutrient with it. Figure 2.10 illustrates this point. Plants obtain mobile nutrients from a “root system sorption zone” and are capable of using nearly all of the mobile nutrient (or mobile form of the nutrient) if the supply is limited. According to Bray, the mobile nutrients are: nitrogen, sulfur, boron and chlorine.
Figure 2.10. The large volume of soil from which plants extract mobile nutrients (root system sorption zone).
In a field situation, where there is more than one plant, root system sorption zones overlap if plants are close enough together as illustrated in Figure 2.11. As a result there is a volume of soil between plants where the nutrient is in demand by both plants. As plants are placed closer and closer together (e.g. increasing plant population to increase potential yield) the competition for nutrients increases. Unless the competition among plants in a field for a mobile nutrient is satisfied by supplying more of the nutrient, the growth and yield of plants will be restricted. From this simple illustration we learn the supply of mobile nutrients like nitrogen must be provided in direct proportion to the number of plants, or potential yield of the crop. This “supply” can be easily determined by calculating the amount of nutrient that will be taken up by the crop. To do this, we only need to know the average concentration of the nutrient in the crop and what the crop yield will be. Average nutrient concentrations are commonly known, however yields vary from field to field and year to year. For this reason it is critical to have in mind a “yield goal” or expected yield in order to determine fertilizer needs for mobile nutrients like nitrogen. For example, in Oklahoma the rule “2 pounds nitrogen per acre for every bushel of wheat” is commonly used to estimate the nitrogen requirements of winter wheat. This rule takes into account that soil test and fertilizer nitrogen will only be about 70 percent utilized by the plant. Because mobile nutrients are almost completely extracted from the root system zone, soil test values like nitrate nitrogen will change drastically from one year to the next in relation to how much nitrogen was available and the crop yield.
Figure 2.11. Competition among plants brought about by increasing yield goal.
Immobile Nutrients
Nutrients that are immobile in the soil are: phosphorus, potassium, calcium, magnesium, iron, zinc, manganese, copper and molybdenum. These nutrients are not transported to plant roots as soil water moves to and is absorbed by the root. These nutrients are absorbed from the soil and soil water that is right next to the root surface. Because of this there is only a small volume of soil next to the root surface that is involved in supplying immobile nutrients to plants. Figure 2.12 identifies this soil volume as the root surface sorption zone. This figure illustrates that only a small fraction of the soil in the total rooting zone is actually involved in supplying immobile nutrients. The total amount of immobile nutrient in the whole soil volume is not as important as the concentration right next to the root surface. Because only the thin layer of soil surrounding the roots is involved in supplying immobile nutrients, when more plants are considered as in Figure 2.13, there is still little or no competition among the plants for immobile nutrients. Competition would occur only at points where roots from adjacent plants actually came in contact with one another. This illustration indicates that the supply of immobile nutrients like phosphorus does not have to be adjusted (increased) in relation to an increase in yield goal or yield potential. If soil availability is adequate for a 25-bushel wheat yield, then in the event that conditions are favorable (better moisture supply) for 50-plus-bushel yield, the more extensive root system that develops for the higher yield will explore new soil and extract the required phosphorus.
Figure 2.12. Small volume of soil from which plants extract immobile nutrients (root surface sorption zone).
Figure 2.13. Limited competition among plants for immobile nutrients.
The mobility concept and these simple illustrations can help one understand the basis for some common practices and observations. For example, immobile nutrient fertilizers usually are more effective if they can be incorporated, but especially should be placed where roots have a high probability of coming in contact with the fertilizer. This is why band applying phosphate fertilizers is generally more effective than the same rate broadcast and incorporated. Mobile nutrients like nitrogen can be broadcast during the growing season (topdressing wheat) because they are moved easily to the roots with rain or irrigation. The phosphorus soil test does not change much from year to year regardless of the previous year’s yield or fertilizer rate because much of the soil was not in contact with the roots or fertilizer and its available phosphorus status was therefore unchanged. Continued broadcast application of high rates of phosphorus will cause a build up and an increase in the soil test phosphorus because only a fraction (15 to 20 percent) of the fertilizer comes in contact with the roots (fertilizes the crop) and the rest reacts only with the soil (fertilizes the soil).
It sometimes is useful to compare mobile and immobile nutrients and their management to fuel and oil for a tractor or pickup. Fuel is required in relation to the amount of work expected from the tractor in much the same way nitrogen is required in relation to the amount of yield expected from the crop. Oil is required more in relation to the level in the crankcase identified by the dipstick than by what or how much work is expected from the tractor (oil burners excepted). Similarly, phosphorus and potassium requirements are determined from the soil test and the amount of fertilizer recommended does not depend on the yield goal. Like the dipstick that is calibrated with a mark showing full and 1-quart low, the soil test for phosphorus (and any immobile nutrient) must be calibrated by field research. Just as the dipstick is uniquely calibrated for each kind of tractor, soil test calibrations vary slightly for different crops and soils and may be somewhat unique for states and regions.
Figure 2.14. Nitrogen cycle.
Advanced Considerations
The students and faculty at OSU developed a nitrogen cycle (Figure 2.14) that includes various components interlinked with what has been presented here. In addition, this cycle includes the relationships of temperature, pH and oxygen with nitrogen dynamics in plant-soil systems. Note that this cycle is more complex than that illustrated in Figures 2.4, 2.6 or 2.7.
Chapter 3 - Problem Soils
Hailin Zhang
Most soils in Oklahoma have developed under conditions that have resulted in them being naturally productive. Because of how they have been managed for agricultural production and otherwise changed by man’s activities, some of these soils are now less productive. Two of the most common causes of productivity losses are the development of acidic and saline (including saline-alkali and alkali) conditions. They are often considered as problem soils because they do not respond to normal management. Therefore, their treatment and management should be different.
Acid Soils
Soil acidity is a crop production problem of increasing concern in central and western Oklahoma. Although acid soil conditions are more widespread in eastern Oklahoma, their more natural occurrence has resulted in farm operators being better able to manage soil acidity in that part of the state. However, in central and western Oklahoma this problem is increasing with time.
The median soil pH of all agricultural samples tested by the Soil, Water and Forage Analytical Laboratory from 2009 to 2013 was 6.1. This means 50 percent of the sample had a pH less than 6.1 and 50 percent higher than 6.1 statewide. Some counties had more than 35 percent of fields with pH lower than 5.5, which is critically low for most field crops. The median soil pH for all counties is shown in Figure 3.1. More acidic soils frequently are found in the central part of the state, which likely is due to intensive crop production.
Figure 3.1: Median soil pH for all Oklahoma counties tested between 2009 and 2013.
Why Soils are Acidic
The four major causes for soils to become acidic are listed below:
- Rainfall and leaching
- Acidic parent material
- Organic matter decay
- Harvest of high yielding crops
- Nitrification of ammonium
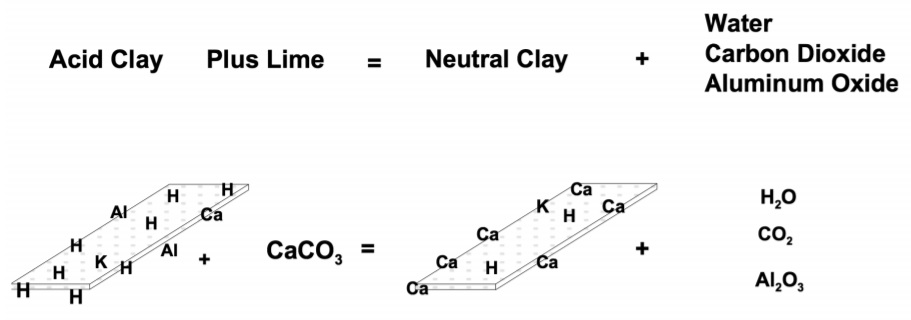
The above causes of soil acidity are most easily understood when we consider a soil is acidic when there is an abundance of acidic cations, like hydrogen (H+) and aluminum (Al3+) present compared to the alkaline cations like calcium (Ca2+), magnesium (Mg2+), potassium (K+), and sodium (Na+).
Rainfall and Leaching
Excessive rainfall is an effective agent for removing basic cations. In Oklahoma, for example, we generally can conclude soils are naturally acidic if the rainfall is above about 30 inches per year. Therefore, soils east of I-35 tend to be acidic and those west of I-35, alkaline. There are many exceptions to this rule though, mostly as a result of item 4, intensive crop production and application of nitrogen fertilizers. Rainfall is most effective in causing soils to become acidic if a lot of water moves through the soil rapidly. Sandy soils are often the first to become acidic because water percolates rapidly, and sandy soils contain only a small reservoir (buffer capacity) of bases due to low clay and organic matter contents. Since the effect of rainfall on acid soil development is very slow, it may take hundreds of years for new parent material to become acidic even under high rainfall.
Parent Material
Due to differences in chemical composition of parent materials, soils will become acidic after different lengths of time. Thus, soils that developed from granite material are likely to be more acidic than soils developed from calcareous shale or limestone.
Crop Production
Harvesting of crops has its effect on soil acidity development because crops absorb lime-like elements, as cations, for their nutrition. When these crops are harvested and the yield is removed from the field, some of the basic material responsible for counteracting the acidity developed by other processes is lost, and the net effect is increased soil acidity. Increasing crop yields will cause greater amounts of basic material to be removed. Grain contains less basic materials than leaves or stems. For this reason, soil acidity will develop faster under continuous wheat pasture than when only grain is harvested. High yielding forages, such as Bermudagrass or alfalfa, can cause soil acidity to develop faster than with other crops.
Table 3.1 identifies the approximate amount of lime-like elements removed from the soil by a 30-bushel wheat crop. Note there is almost four times as much lime material removed in the forage as the grain. This explains why wheat pasture that is grazed will become acidic much faster than when grain alone is produced. Using 50 percent Effective calcium carbonate equivalent lime, it would take about one ton every 10 years to maintain soil pH when straw (or forage) and grain are harvested annually at the 30-bushels-per-acre level.
Nitrification
The use of fertilizers, especially those supplying nitrogen, often is a cause of soil acidity. Acidity is produced when ammonium containing materials are transformed to nitrate in the soil. The more ammoniacal nitrogen fertilizer is applied, the more acidic the soil gets.
Table 3.1: Bases removed by a 30-bushel wheat crop.
(CALCIUM CARBONATE EQUIVALENTS)
|
|
Calcium |
Potassium |
Magnesium |
Sodium |
Total |
|---|---|---|---|---|---|
|
Grain |
2 |
10 |
10 |
2 |
24 |
|
Straw* |
11 |
45 |
14 |
9 |
79 |
|
Total |
13 |
55 |
24 |
11 |
103** |
*Straw/forage
**One ton of alfalfa will remove slightly more than this amount.
What Happens in Acid Soils
Knowing the soil pH helps identify the kinds of chemical reactions likely to occur in soils. Generally, the most important reactions, from the standpoint of crop production are those dealing with solubilities of compounds or materials in soils. In this regard, we are most concerned with the effects of pH on the availability of toxic elements and nutrient elements.
Toxic elements like aluminum (Al) and manganese (Mn) are the major causes for crop failure in acid soils. These elements are a problem in acid soils because they are more soluble (available for plant uptake) at low pH. In other words, more of the solid form of these elements will dissolve in water when the pH is very low. There is always a lot of solid aluminum present in soils because it is a part of most clay particles.
Element Toxicities
When soil pH is above 5.5, aluminum in soils remains in a solid combination with other elements and is not harmful to plants. As pH drops below 5.5, aluminum containing materials begin to dissolve. Because of its nature as a trivalent cation (Al3+), the amount of dissolved aluminum is 1,000 times greater at pH 4.5 than at 5.5 and 1,000 times greater at 3.5 than at 4.5. For this reason, some crops may seem to do very well, but then fail completely with just a small change in soil pH. Wheat, for example, may do well even at pH 5.0, but usually will fail completely at a pH of 4.0.
The relationship between pH and dissolved manganese in the soil is similar to that
described for aluminum, except that manganese (Mn2+) only increases 100 fold when the pH drops from 5.0 to 4.0.
Toxic levels of aluminum harm the crop by root pruning. That is, a small amount of aluminum in the soil solution in excess of what is normal causes the roots of most plants to either deteriorate or stop growing. As a result, the plants are unable to normally absorb water and nutrients, appear stunted and exhibit nutrient deficiency symptoms, especially those for phosphorus. The final effect is either complete crop failure or significant yield loss. Often, the field will appear to be under greater stress from pests, such as weeds, because of the poor crop conditions.
Toxic levels of manganese interfere with normal growth processes in above ground plant parts. This usually results in stunted, discolored growth and poor yields.
Desirable pH
The adverse effect of these toxic elements is most easily (and economically) eliminated by liming the soil. Liming raises soil pH and causes aluminum and manganese to go from the soil solution back into solid (non-toxic) chemical forms. For grasses, raising soil pH to 5.5 will generally restore normal yields. Legumes, on the other hand, do best in a calcium-rich environment and often need a soil pH between 6.5 and 7.0 for maximum yields.
Soil pH in the range of 6.0 to 7.0 also is desirable from the stand point of optimum nutrient availability. However, the most common nutrient deficiencies in Oklahoma are for nitrogen, potassium and phosphorus, and availability of these elements will not be greatly changed by liming. Nutrients most affected by soil pH are iron and molybdenum. Iron deficiency is more likely to occur in non-acid (high pH) soils. Molybdenum deficiency is not common in Oklahoma, but would be most apt to occur in acid soils and could be corrected by liming.
Soil Buffer Capacity and Buffer Index
Although crops remove large quantities of lime-like materials that are harvested each year, the soil pH usually does not change noticeably from one season to the next. Because soil pH does not change quickly, it is said to be buffered. Buffer means the resistance to the change of pH.
There are several reasons why soils have this buffer ability or capacity. For example, in the Oklahoma Panhandle, soils commonly contain free calcium carbonate (lime). The term caliche is used to describe layers of soil material cemented by accumulated calcium carbonate. These accumulations provide a huge reserve of lime that will maintain soil pH in the alkaline range (above pH 7.0) for generations, perhaps centuries, even with the most productive agricultural systems.
A second contribution to the buffering capacity of soils is the release of basic chemical elements from normal chemical weathering of soil minerals. This is a very slow process that occurs whenever water is added to soil. The effect is influenced by the type of minerals in the soil, the amount and frequency of water addition, and soil temperature.
The most important source of buffer capacity in acid soils (no free lime present) is exchangeable cations. These are the lime-like chemical elements (mostly calcium) that are adsorbed on the surface of soil particles. These adsorbed basic materials act like a large reservoir that continually replenishes basic materials in the soil solution when they are removed by a crop or neutralized by acid. Figure 3.2 illustrates this and the relationship between soil pH and buffer capacity.
As crops remove bases from soil water in the reservoir on the right (Figure 3.2), bases from the large reservoir of soil solids (clay and humus) on the left move to the soil solution and replenish the supply. Because of this relationship and the large reserve of bases from soil solids, the pH does not change much from month to month or even year to year. Also since the large reservoir on the left is shaped like a pyramid, pH can often be changed more easily by liming at pH near 6 than in the very acid pH 4.5 to 5.5 range.
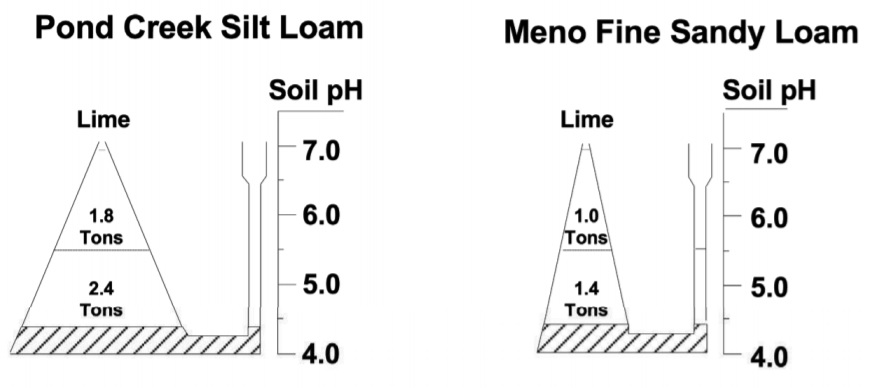
Figure 3.3 shows the influence of soil organic matter and texture on buffer capacity. Both soils have a pH of 4.3, and are too acidic for efficient crop production. In order to provide a more favorable pH, the soils must each be limed. The amount of lime required will depend on the size of the large reservoirs and how base depleted they may be.
From these diagrams it is easy to understand why it takes much more lime to raise the pH of a clay soil with its large reservoir than it does for a sandy soil and its small reservoir. Also, because the reservoir of sandy soil is small, if acidifying conditions are equal, sandy soil will tend to become acidic more rapidly and need to be limed more frequently than a clayey soil.
Figure 3.2: The relationship of basic materials in soil solids to pH of the soil solution.
Figure 3.3: Reservoirs of soil solids in clayey vs. sandy soil.
The Soil Test
Buffer Index, measured in the laboratory as a part of the OSU routine soil test, is an indirect estimate of the soil reservoir size for storing basic material. Because the test involves adding basic (lime-like) material to soils of pH less than 6.3 and then measuring pH again, the BI pH is larger when the reservoir is small. The two soils illustrated in Figure 3.3 need to be limed. The Pond Creek Silt Loam soil would have a Buffer Index value of about 6.2. About 4.2 tons of effective calcium carbonate equivalent lime would be required to raise the soil pH to 6.5. The sandy soil, having the same soil pH, would have a BI value of about 6.5 and require only 2.5 tons of effective calcium carbonate equivalent lime to reach the same pH. The field calibration for BI and lime requirement is provided in Table 3.2.
How to Interpret pH and Buffer Index
Considering a soil test result of pH 5.8 and Buffer Index 6.8, where establishment of alfalfa is intended, the following steps are taken to determine the lime requirement.
First, the soil test pH of 5.8 is compared to the preferred pH for alfalfa in Table 3.3. Since the soil pH 5.8 is below the lowest pH in the preferred range, lime must be added to raise the pH to the desired level.
The amount of lime required is determined from Table 3.2 by locating the Buffer Index value of 6.8 in the left hand column and matching it to the number directly across from it (bold) under the middle column of numbers. In this case, 1.2 tons of effective calcium carbonate equivalent lime would be required.
If the intended crop is wheat instead of alfalfa, no lime is required because Table 3.3 shows that pH 5.8 is satisfactory for wheat production. Since the pH is satisfactory for wheat, the lime requirement would not be reported, even though the Buffer Index was measured. It would be important to regularly test this soil, especially if it were sandy, so lime could be applied before the soil became seriously acid (below pH 5.0) for wheat production.
Remember, the Buffer Index is used only as a guide for how much lime should be added to an acid soil when it is necessary to raise soil pH.
Table 3.2: Tons of effective calcium carbonate equivalent* lime required to raise soil pH of a 6-7 inch furrow slice to pH 6.5 or 6.4.
(LIME REQUIRED)
| Buffer Index | All other crops | Continuous wheat |
|---|---|---|
|
7.2+ |
0 |
0 |
|
7.1 |
0.5 |
0.5 |
|
7 |
0.7 |
0.5 |
|
6.9 |
1 |
0.5 |
|
6.8 |
1.2 |
0.6 |
|
6.7 |
1.4 |
0.7 |
|
6.6 |
1.9 |
1 |
|
6.5 |
2.5 |
1.3 |
|
6.4 |
3.1 |
1.6 |
|
6.3 |
3.7 |
1.9 |
|
6.2 |
4.2 |
2.1 |
*Effective calcium carbonate equivalent guaranteed by lime vendor.
Table 3.3: Common pH preference of field crops.
| Crops | pH Range | |
|---|---|---|
| Legumes | ||
| Cowpeas, Crimson Clover, | ||
| Mungbeans and Vetch | 5.5-7.0 | |
| Soybeans, Peanuts | ||
| Alsike, Red and White, | 5.8-7.0 | |
| (Ladino) Clovers,and | ||
| Arrowleaf Clover | 6.0-7.0 | |
| Alfalfa and Sweet Clover | 6.3-7.5 | |
| Non-Legumes | ||
| Fescue and Weeping Lovegrass | 4.5-7.0 | |
| Buckwheat | 5.0-6.5 | |
| Sorghum, Sudan, Corn and Wheat | 5.5-7.0 | |
| Bermuda, Canola | 5.7-7.0 | |
| Barley | 6.3-7.0 |
Correcting Soil Acidity
Lime Reactions
Soil acidity can be corrected only by neutralizing the acid present, which is done by adding a basic material. While there are many basic materials that can neutralize acids, most are too costly or difficult to manage. The most commonly used material is agricultural limestone (aglime). It is used because it is relatively inexpensive and easy to manage.
The reason limestone is easy to manage is because it is not very soluble, meaning it does not dissolve easily in water. For this reason, it is not very corrosive to equipment, and more importantly, its pH at equilibrium (after it has dissolved as much as it can and there is still some lime left in the water) is only about 8.3. This latter aspect is very important because even if an excessive amount of lime is applied, a harmful effect on crop yields would generally not take place.
The reaction of lime, or calcium carbonate (CaCO3), with an acid soil is illustrated by Figure 3.4.
This diagram shows that the acidity is on the surface of soil particles. As lime dissolves in the soil, calcium from the lime moves to the surface of soil particles and replaces the acidity (H+ and Al3+). The acidity reacts with carbonate (CO3) to form carbon dioxide (CO2), water (H2O) and insoluble Al. The end result is a soil that is less acid.
Figure 3.4: Illustration of how aglime neutralizes soil acidity.
Lime Research
Several field research experiments have been conducted on wheat in the past to examine suitable liming materials and application rates. A common feature of all effective commercially available liming materials is that they contain a basic lime-like material such as calcium or magnesium carbonate. Since it is ultimately the material from which other basic materials are derived, aglime is usually the lowest cost per ton of active ingredient (effective calcium carbonate equivalent, finely ground pure CaCO3 is defined to have an effective calcium carbonate equivalent of 100).
A long-term liming study on wheat was conducted during a nine-year period on a Pond Creek silt loam soil near Carrier, Oklahoma. Results of the study are illustrated in Figure 3.5 and show through nine harvests, the yield of wheat was greatly improved by a single application of lime. It is important to note although 4.8 tons of effective calcium carbonate equivalent lime were recommended from the soil test in order to raise the pH to 6.8, one-fourth that rate (only 1.2 ton effective calcium carbonate equivalent) was sufficient for eight years to restore yields to almost 100 percent of the yield obtained when 4.8 tons effective calcium carbonate equivalent were applied. The 2.4 tons effective calcium carbonate equivalent rate, one-half the normally recommended rate, was still effective at the end of the experiment.
Figure 3.5: Long-term effect of lime on wheat yields.
Using information from field studies, such as the Carrier site, a relationship between OSU soil test pH values and expected wheat yield has been developed (Figure 3.6). The yield at a given pH is expressed as relative yield. This term means the expected yield as a percentage of that possible if soil acidity was not a limiting factor. For example, if a 40-bushel yield is expected with no acidity problems then at a soil pH of 5.0 a relative yield of 85 percent or 34 bushels, would be expected.
Figure 3.6: The effect of soil pH on wheat yields.
Lime Rates
(Minimum Amounts)
The amount of lime to apply for wheat production depends on whether you are growing continuous wheat or will rotate wheat with a legume. If wheat alone is grown year after year, it is necessary to only apply a rate of lime to raise the pH to above 5.5 because higher pH may favor some root rot diseases. If legumes are sometimes grown, then soil pH should be raised to 6.5 or above. Thus, for continuous wheat the following recommendation is made: The minimum amount of lime to apply is 0.5 ton effective calcium carbonate equivalent lime or 50 percent of the soil test deficiency amount required to raise the pH to 6.5, whichever is greater. An OSU soil test will identify these lime rates for wheat whenever the soil pH is below 5.5.
Calculating Rates
Lime requirements are expressed in terms of Effective calcium carbonate equivalent. The Effective calcium carbonate equivalent is provided as a guarantee from lime vendors who are registered to sell aglime in Oklahoma. The guarantee is obtained by an analysis of the lime by the Oklahoma State Department of Agriculture, Food and Forestry. There are two components to the determination by their lab. First, the purity of the lime is determined chemically (purity factor). In this test they analyze for the fraction of CaCO3, or its equivalent, in the lime material. The second measurement is a determination of how finely the lime particles are ground (fineness factor). The fineness factor is determined by weighing sieved portions of a lime sample. The factor is then calculated by taking one-half times the fraction (e.g. 0.90) of sample passing an 8 mesh sieve plus one-half times the fraction (e.g. 0.70) of sample passing a 60 mesh sieve. The fineness factor for these example values would be:
.5 x 0.90 + .5 x 0.70 = 0.80
The purity factor (a fraction) and the fineness factor (a fraction) are multiplied by 100 to obtain the effective calcium carbonate equivalent value. If the purity factor was 0.90 (90 percent pure or equivalent calcium carbonate) then the effective calcium carbonate equivalent would be (0.90 x 0.80) x 100, or 72 percent. The more CaCO3 in the material and the finer the particle size, the greater the effective calcium carbonate equivalent. Good quality lime will have an effective calcium carbonate equivalent value above 60 percent. Because aglime does not always have an effective calcium carbonate equivalent of 100 percent, the amount required to provide a given amount of 100 percent effective calcium carbonate equivalent must be calculated. The calculations to use are shown below:
Effective calcium carbonate equivalent lime required x 100 = aglime required
Divided by
% Effective calcium carbonate equivalent
For example, let us assume the available aglime was 72 percent effective calcium carbonate equivalent and the soil test indicated a need for 1.5 tons effective calcium carbonate equivalent to raise the soil pH to the desired level.
The calculations would be:
(1.5 x 100) / 72 = 2.1 tons of aglime
So, 2.1 tons per acre of the 72 percent effective calcium carbonate equivalent lime would have to be applied in order to get the 1.5 tons of 100 percent effective calcium carbonate equivalent lime required to do the job.
Lime Applications
Because lime does not dissolve easily in water, it must be treated similarly to fertilizers that supply the soil with immobile nutrients like phosphorus. Thus, for lime to be most effective in neutralizing soil acidity it must be thoroughly mixed with the soil. Since neutralization involves a reaction between soil particles and lime particles, the better lime is mixed with the soil, the more efficiently the acidity is neutralized. For this reason, wet materials (like that from water treatment plants) which cannot be thoroughly mixed with the soil are often less effective. Similarly, pelleted lime particles are too large to mix well with small soil particles. Attempts to mix these materials with soil often result in soil acidity being neutralized only near the lime aggregates (or pellets), whereas acidity between aggregates remains unaffected. Once the proper rate has been determined and the lime has been spread to give a uniform application over the field, it is best to incorporate it with a light tillage operation such as disking. Disking can be followed by plowing, but care should be taken not to plow too deeply or the lime will be diluted by subsoil and be less effective. Lime rates are calculated on the basis of neutralizing the top six inches of soil.
Since the lime reaction involves water, the effect of lime will be very slow in dry soil. Even when everything is done correctly and the soil is moist, it often takes a year or more for a measurable change in soil pH to occur. For this reason, liming for wheat production should be done as soon after harvest as possible. However, when the soil pH is extremely low, sufficient change may occur in just a few weeks and make the difference between being able to establish a wheat crop and having a failure.
A similar approach should be used for annual planting of other grasses. When continuous production of perennial grasses is planned, the full rate identified by the soil test buffer index should be applied pre-plant. This practice allows incorporation of the lime to maximize its reaction with soil and will maintain a desirable pH for several years after establishment. Careful monitoring of high producing forage grasses, such as Bermudagrasses, by periodic soil testing will identify lime needs early enough to maintain desirable soil pH by unincorporated broadcast application.
Liming Materials
The most common and most effective liming material continues to be ground aglime. It is marketed by the ton, should generally be powdery with only a small percentage of coarse (sand size) particles, and have an effective calcium carbonate equivalent of 50 percent or greater. Variations and different formulations of ground aglime have been developed and marketed. These materials often are promoted on the basis of being more effective or less expensive. The merits of these products should be considered carefully.
“Liquid Lime” is a formulation of high-quality aglime (effective calcium carbonate equivalent above 90 percent) with water and enough clay to keep the lime in suspension. The amount of water added may range from 35 to 50 percent. Care should be taken to make sure that the added water is not being charged for, as if it were high quality lime. When 90 percent effective calcium carbonate equivalent lime is mixed 50 percent (weight to weight) with water, the resulting product is only 45 percent effective calcium carbonate equivalent lime (90 percent x .50 = 45 percent). The fact that it is suspended in water does not increase its effectiveness. On the contrary, wet lime will not mix as easily with soil and therefore, its neutralizing effectiveness may be less than an equal amount of dry effective calcium carbonate equivalent aglime.
Similarly, “water treatment lime” may not be as effective as an equal rate of aglime. This material is a waste product from water treatment plants. Although it has a high Effective calcium carbonate equivalent, it often is wet when applied and a good mixture with soil is difficult to obtain. Too often, large chunks or globs remain mixed with the soil and only the acid soil next to the chunk of lime is neutralized, leaving large areas of soil between chunks that remain acid.
Pelleted lime is finely ground lime pressed into pellets. Until the pellets physically break up and the fragments of powder size lime become thoroughly mixed with soil, these too are limited in neutralizing soil acidity. Pellets, liquid lime, and water treatment lime can be spread or applied without dust common to good aglime. Although easily visible, airborn dust associated with aglime application represents only a small fraction of the total applied, and loss from the field should not be significant.
Finally, sometimes coarse road grade lime is in abundance and can be purchased at a very low cost. This cheap lime is too coarse to have a reasonable effective calcium carbonate equivalent and will not be sold as aglime. Because of the existing aglime law in Oklahoma, whenever a material is marketed and sold in Oklahoma as aglime it must be accompanied by a guaranteed effective calcium carbonate equivalent. The guaranteed effective calcium carbonate equivalent must be of the formulated product and not its ingredients.
Reducing Metal Toxicity
Fertilizer Reactions
Phosphate in the soil has long been known to be less available to crops in some extremely acid soils because it reacts with aluminum and/or manganese, which are more available in acid soils. When phosphate reacts with these metals, the compound formed is a very insoluble solid (such as aluminum phosphate). As a result, not only is the phosphate unavailable, but also the aluminum and manganese are unavailable. For these reasons, when phosphate fertilizers are banded with the seed at planting time, the harmful effects of toxic aluminum and manganese are greatly reduced, and near-normal yields may be obtained. Figure 3.7 illustrates the benefit of this practice for both grain and forage production.
Figure 3.7. Responses of wheat grain and forage yields to seed-applied phosphate fertilizers (APP: ammonium polyphosphate; DAP: diammonium phosphate in a strongly acidic soil.
Phosphate Materials and Rates
Figure 3.7 also shows a higher rate of phosphate may be needed in order to get maximum benefits for fall forage production. It is especially important to use the higher rate for forage production on soil that has a pH below 4.5. The use of phosphate fertilizer in this way does not change soil pH. Also, within a few months after all the phosphate has been used up, more aluminum and manganese may become available. While this may not affect the developed crop, it will affect the next crop in the seedling stage. As a result, phosphate fertilizer must be applied each year whereas lime only needs to be applied every five to eight years. On the other hand, buildup of soil test phosphorus above crop needs may lead to increased phosphorus in the runoff.
When to Use Phosphate
As stated earlier, acid soil is best neutralized by adding aglime. However, seed-applied phosphate (either ammonium polyphosphate or diammonium phosphate) should be considered for acid wheatland soils when:
- the land is owned by someone who will not provide a long-term lease or pay some of the cost for liming,
- the soil acidity problem is discovered too late for lime application in a given season or,
- the soil has a low soil test value for phosphorus.
It is important to remember this use of phosphate fertilizer is very different from normal. Banding phosphate on acid soils can increase yields even when the phosphate soil test value is high (more than 65), not because more phosphate is provided to the plant, but because metal toxicity is reduced. Also, it is important to remember the soil continues to become more acidic with time. Eventually, lime must be added to the soil to neutralize acidity
Saline and Alkali Soil
Two other problem soils are salty (saline) soils and slick-spot (alkali or sodic) soils. A third problem soil often develops from slick spots when they are poorly managed. This is the saline-alkali soil which results when slick-spot soils become salty.
Although all problem soils may be identified by poor crop production, these soils have other similarities and differences that are important to know before attempting to improve or reclaim them.
Saline soils are soils that contain at least 2600 parts per million dissolved salts in the solution from a soil saturated with water. The salt content is estimated by laboratory measurement of how well the soil water conducts electricity, and saline soils are those with an electrical conductivity (EC) of 4,000 micromohs/cm (about 2,600 parts per million total dissolved salt). This level of salts is great enough to reduce production of salt-sensitive crops. Normal, productive agricultural soils commonly have electrical conductivity values below 1,000.
Alkali soils are soils which contain enough sodium to cause 15 percent of the cation exchange sites to be occupied by sodium. Sodium in the soil prevents clay particles (and other very small, colloidal sized particles such as humus) from coming together and forming large soil aggregates. When soils contain 15 percent or more of exchangeable sodium most of the clay and humus particles are unattached or dispersed. These soils commonly have a pH of 8.5 or above (alkali). Some Oklahoma soils become dispersed when the exchangeable sodium is as low as 7 percent. Productive agricultural soils often have less than 1 percent exchangeable sodium. Soils can be classified into 4 groups based on the EC and ESP of saturated paste extract. They are illustrated in Figure 3.8.
Figure 3.8. General classification of salt affected soils.
Characteristics of Saline Soils
Small, Growing Areas Affected
Naturally developed saline soils usually represent only small areas of a field. Often these are low-lying parts of the field that may have poor internal soil drainage. Other small areas occur on slopes where erosion has exposed saline or alkali subsoil. Because low areas frequently are wet when the rest of the field is dry enough for cultivation, these small areas frequently are cultivated when the soil is too wet. This results in the soil becoming compacted in and around the area. Water does not move easily through the compacted soil so more water evaporates, leaving salts from the water to accumulate. As a result, the affected area increases with time.
Poor Yield
Crop production usually is less than normal in salt affected areas. Yield reduction is greatest in years of less than normal rainfall or when water stress has been a yield limiting factor. Salts tie up much of the water in the soil and prevent plants from absorbing it. Seedlings are the most sensitive to water stress and crop stand is reduced because of seedling death and poor yield results.
White Surface Crust
As water evaporates from saline soils, salts in the water are left behind to accumulate on the soil surface. Salts are light colored and when accumulation has continued for several days they form a very thin white film on the soil surface. During hot, dry weather, the light film will show up first along edges of the salt problem areas. The center of these areas usually has the most salt and will dry out last.
Good Soil Tilth
Saline soils generally have excellent physical conditions throughout the tillage depth. This is caused by salts effectively neutralizing the negative charge of clay particles, allowing them to attach to one another. When these soils are not too wet, the soil is friable, mellow and easily tilled.
High Soil Fertility
Soil that has been saline for several years usually will be very fertile, and high nitrogen, phosphorus and potassium soil test values are often a clue of a problem salty soil. These nutrients build up in salty areas when there is little crop nutrient removal and the area is fertilized each year. Soil pH does not change in relation to salt content and it cannot be used as an indicator.
Characteristics of Alkali Soils
Except as noted, alkali soils have characteristics similar to saline soils. For this reason, one problem soil may be confused with another. Their differences, however, are important to note as they relate to correcting the problem soils.
Poor Soil Tilth
The excess sodium in alkali soils does not allow soil particles to easily attach to one another. As a result, alkali soil dispersed and not friable or mellow like saline soil. Instead, alkali soil is slick-spot soil that is greasy when wet, especially if it is fine textured, and often very hard when dry. This poor physical condition makes these soils difficult to manage. They often are either too wet or too dry for tillage. Poor seed germination and stand establishment are common because good seedbed preparation is seldom accomplished. As a result, yields usually are lower than the rest of the field and fertility may build up.
Dark- or Light-Colored Surface
Soil colloids floating in the soil water are left as a thin film on the surface after water evaporates. The surface color will be darker than the rest of the field (black-alkali) when the particles are mainly humus since humic acid dissolves in alkali solution and lighter (white-alkali) when the particles are mainly clay and salts. The salts show up as a film when the surface dries.
Droughty Water
Large pores or channels in the soil which allow water entry and penetration become plugged with dispersed clay and humus. As a result, the subsoil may be very dry even though water is ponded on the surface. Plants that become established often suffer water stress and may eventually die from lack of water and/or oxygen.
Reclamation
In many instances, saline soils and alkali soils can be reclaimed by following a definite series of management steps designed to leach or wash out the salts or sodium. The order and description of these steps follows.
Verify Problem
The first step to solving the problem is clearly identifying it. This is best done by having the soil tested. Suspected areas should be sampled separate from the rest of the field. It is best to sample during a dry period of the growing season when affected areas of the field can easily be identified by poor crop growth. Samples should be taken at least one week from the last rain or irrigation and only the top three inches of soil should be sampled. Several small samples of the affected area should be combined in a plastic bucket and mixed to get a good sample.
About one pint of soil is required for the test which is done by the OSU Soil, Water and Forage Analytical Laboratory. Samples should be submitted through your County Extension Office requesting a salinity management test. Testing takes about a week and a small fee is charged to cover costs. This test will identify the type and severity of the problem.
Identify Cause
Whenever possible, it is important to find out what has caused the problem soil to develop. Knowing the cause can help in modifying the remaining reclamation practices and sometimes provide a clue as to how long it may take to complete the reclamation. The four most common causes of saline and alkali soils in Oklahoma are
- naturally poor drainage,
- poor irrigation water,
- brine spills and
- exposure of saline or alkali subsoil due to erosion.
Poorly drained soils are simply soils which water does not easily penetrate. This condition may be a result of the soil having a high clay content, having a water table near the surface (within 10 feet) or existing in a low-lying area of the field. In the last situation, normally adequate internal drainage may not be able to handle runoff from the surrounding area. In some instances, internal soil drainage is reduced greatly as a result of compacting the surface soil.
Use of poor-quality irrigation water may cause problem soils to develop if special precautions are not taken. The problem develops most rapidly during extremely dry years when evaporation and the amount of irrigation are high. Internal soil drainage also may be a contributing factor.
Problem soils sometimes develop seemingly overnight when brine solutions associated with oil- and gas-well activities spill onto the soil. Depending on the amount of brine solution spilled and the size of the area, the problem may be slight or very severe. Whenever the source of salt or sodium causing the problem is the result of addition from runoff, seeps, irrigation water or spilled brine, it is important to eliminate that source as soon as possible.
Improve Internal Soil Drainage
There are no chemicals or soil amendments that can be added to the soil to tie up or somehow inactivate soluble salts or sodium. Hence, the only way of lowering their concentration in the soil is to remove them. This can only be done by leaching (washing out) the salt or sodium downward out of the root zone. In order for this to happen, internal drainage must be good so water can easily pass through the soil.
There are a number of ways internal drainage can be improved. Most are expensive, but when the problem is severe many will pay for themselves with time. Tile drains and open ditches are effective for removing subsoil water that accumulates due to a restrictive layer such as compacted clay or bed rock. Compacted soil layers near the surface can be broken up by subsoiling. This is effective only if done when the soil is dry enough to have a shattering effect and at best provides only temporary benefit.
Problem soils which have developed from use of poor irrigation water or brine spills may already have good internal soil drainage.
Add Organic Matter
Once internal drainage has been assured, the next important step is to improve water movement into the soil. Incorporating 20-30 tons per acre of organic matter into the top six inches of soil creates large pores or channels for water to enter. Even rainfall from intense storms is more effective because there is less runoff. In addition to improving water movement into the soil, the large pores lessen the capillary or wick-like upward water movement during dry periods. Any coarse organic material such as barnyard manure, straw, rotted hay or crop residue is suitable.
Add Gypsum to Slick Spots
Up to this point the reclamation practices are the same for both saline and alkali soils. In either situation, leaching is critical to remove salt or sodium. However, since high amounts of sodium absorbed to the soil are the cause of alkali problems, sodium must be loosened from the soil before it can be leached out. Gypsum is the most effective soil amendment for removing sodium from the soil particles. Gypsum is a slightly soluble salt of calcium sulfate. This means gypsum will slowly react in the soil, but for a long time. The reaction is illustrated in Figure 3.9.
Figure 3.9: Alkali soil reacting with gypsum to form normal soil.
Gypsum applications are needed when the exchangeable sodium percentage, ESP, approaches 15 percent. Calcium ions (Ca2+) in gypsum replace sodium ions (Na+) on the colloids which results in improved soil physical conditions. The amount of gypsum required will vary widely depending upon the percentage of exchangeable sodium and the soil texture, as determined by the soil test. This relationship is shown in Table 3.4.
When the required amount of gypsum exceeds 5 tons per acre, the rate should be split into two or more applications of no more than 5 tons at one time. Successive applications should not be made until time has allowed for some leaching to occur, and the need has been verified by a second soil test. The gypsum should be incorporated only to a depth of about 1 to 2 inches, which is enough to mix it well with the surface soil and keep it from blowing away. However, if the soil was contaminated by brine spill, the gypsum needs to be mixed to 6 to 7 inches deep to create a favorable rooting zone.
| Texture (Exchangeable Sodium Percentage) (Numbers listed below are gypsum in tons per acre) |
15 | 20 | 30 | 40 | 50 |
|---|---|---|---|---|---|
| Coarse | 2 | 3 | 5 | 7 | 9 |
| Medium | 3 | 5 | 8 | 11 | 14 |
| Fine | 4 | 6 | 10 | 14 | 18 |
Table 3.4. Gypsum requirement in tons per acre as related to soil texture and sodium percentage.
Leach Soil
Leaching (or washing out) the soil is essential to reduce the amount of salts or sodium in the soil. In order for this leaching process to occur, water must enter the soil in excess of what is used by growing crops and lost by evaporation. How fast and to what extent the reclamation is successful will depend on how much good quality water passes through the soil in a given period of time. The shorter the time interval over which excess water is applied, the more effective that amount of water is in reclamation. For this reason, rainfall is most effective when it falls on soil that is already wet.
Avoid Deep Tillage and Establish Cover
Once the leaching process has been started, deep tillage such as moldboard plowing should be avoided for several years to promote uninterrupted downward movement of the salts. Such tillage will bring salt back up to the soil surface, and leaching will be required again. As soon as the salt level in the soil is low enough, a salt-tolerant crop such as barley or Bermudagrass should be established on the problem area to provide a cover for as much of each growing season as possible. It is especially important to have the cover crop during midsummer when evaporation is high. Adequately fertilized Bermudagrass does a good job of drying the soil. To minimize soil compaction it should be cut for hay instead of pastured. Make sure to keep heavy equipment off the area when it is wet.
Some problem areas may be too salty to establish a cover crop until some salts have been leached. A cover crop can be established when there is no longer a white salty film on the soil surface, following a week or two of dry weather, or when weeds begin to grow.
Wait
The final step in reclamation is simply to wait for the previous practices to work. Except for brine spills, these problem soils developed over a period of several years. Reclamation may not take as long, but depending on how well reclamation practices can be carried out, may take one or more years.
Reclamation
Learn to Live With It
The key to successful reclamation is good internal soil drainage. If salts or sodium cannot be leached out, the soil cannot be reclaimed by conventional methods. However, most soils have some internal soil drainage, and although drainage may not be good, over several years time it may be sufficient to lower the salt concentration to near normal. During this time it will be important to practice some of the same steps outlined above. Especially important are the following:
- Avoid excessive fertilization.
- Avoid traffic on field when wet.
- Apply gypsum to slick spots.
- Establish a cover crop.
- Maintain a high level of crop residue.
- Be patient!
Depending on the severity of the problem it may be necessary to select a different crop than has been grown in the past. A list of crops and their relative tolerance to salt is provided in Table 3.5.
Table 3.5: The relative salt tolerance of crops.*
| Tolerant | Moderately Tolerant | Sensitive | |
|---|---|---|---|
| Field Crops | 7,800-10,400 ppm Cotton Sugar beet Barley (grain) |
3,900-7,800 ppm Sunflower Corn Soybeans Grain sorghum Oats (grain) Wheat (grain) Rye (grain) |
2,600 ppm Field beans |
| Forages | 7,800-11,700 ppm Wheatgrass Birdsfoot trefoil Barley (hay) Rescue grass Rhodesgrass Bermudagrass Saltgrass Alkali sacaton |
2,600-7,800 ppm Smooth bromegrass Fescue Blue grama Oats (hay) Wheat (hay) Rye (hay) Alfalfa Sudangrass Dallisgrass Perennial ryegrass Yellow sweetclover White sweetclover |
1,300-2,000 ppm Ladino clover Red clover White Dutch clover Peanuts |
| Vegetable Crops | 6,500-7,800 ppm Spinach Asparagus Kale Garden beets |
2,600-6,500 ppm Cucumber Squash Peas Onion Carrot Bell pepper Sweet potato & yam Potato Sweet corn Lettuce Cauliflower Cabbage Broccoli Tomato |
1,950-2,600 ppm Green beans Celery Radish |
| Fruit Crops | Cantaloupe Grape |
Strawberry Peach Apricot Plum Apple Pear |
*Salt tolerance values at which 50 percent yield reduction may be expected compared to nonsaline conditions. Salt concentrations are from a soil saturated paste extract.
Chapter 4 - Determining Fertilizer Needs
Hailin Zhang
Determining fertilizer and lime needs for selected fields and crops are critical management decisions that often mean the difference between profit and loss for farmers. Applying too little fertilizer or lime when deficiencies exist hurts yield and profit potential. Too much fertilizer reduces nutrient use efficiency, cutting into profits and in some cases, negatively impacting the environment. In today’s economic and political atmosphere, farmers must be concerned about both effects.
At one time, determining fertilizer and lime requirements of Oklahoma crops was simple. If a fertilizer contained phosphate, it was good because almost all Oklahoma soils were low in phosphorus. Because of this, in the early days of fertilizer use, 10-20-10 or 19-19-19 was an effective fertilizer that gained popular use. This thinking no longer applies. Many soils have been fertilized with this practice for many years, increasing soil fertility much above native levels. In other soils, continuous cropping has decreased soil pH values to yield-robbing levels or depleted once abundant supplies of nutrients. Farmers can no longer afford to guess about their fertilizer and lime needs. The fertility levels of each field must be known in order to best manage the entire farm.
There are three approaches to determining fertilizer needs: (1) soil testing, (2) scouting for nutrient deficiency symptoms, and (3) plant analysis. Soil testing is by far the most successful method. To obtain maximum benefit, it must be done on a regular basis and should therefore be viewed as a routine component of an overall soil fertility program. A soil fertility program can be enhanced by scouting for nutrient deficiency symptoms and by using plant analysis when applicable, but soil testing remains as the foundation.
Use of Soil Testing
Soil testing evolved from an understanding by soil scientists that plants require chemical elements as nutrients. Thirteen of the essential nutrient elements for plants come from the soil. The soil’s nutrient-supplying capacity is a chemical characteristic of the soil, and therefore, is most reliably measured or estimated by chemical tests (i.e., soil testing). The concept of soil testing is not new. Even in ancient times, farmers had a limited understanding of basic soil fertility concepts as can be gathered from the ancient agricultural practices documented in Table 4.1. Modernization of soil fertility principles and the refinement of soil testing began in the mid 1800s with advances continuing to this day (Table 4.2).
Table 4.1: Ancient agricultural practices related to soil testing.
| Time | Location | Agricultural Practice |
|---|---|---|
| 2500 B.C. | Mesopotamia | First recorded writings mentioning soil fertility. Barley yields observed to range from 86 to 300 times that planted depending on the area in which the crop was grown. |
| 900 B.C. | Greece | Manuring was an agricultural practice known to improve soil productivity. |
| 300 B.C. | Greece | Various sources of manure were classified according to their value as a soil amendment. Green manure crops, especially legumes, were also known to enrich the soil. |
| 100 B.C | Rome | The value of using marl and other liming materials as soil amendments was recognized. |
| 50 B.C. | Rome | Considered to be when the first soil fertility test was developed. Columella recommended using a taste test to measure the degree of acidity and salinity of soils. |
Table 4.2. Modernization of soil testing.
| Time | Location | Event |
|---|---|---|
| 1842 | Germany | Justus von Liebig stated his “law of the minimum.” |
| 1843 | England | J.B. Lawes and J.H. Gilbert established the Rothamsted Experimental Station. |
| 1892 | U.S.A. | Magruder Plots established by Alexander C. Magruder in Stillwater, Oklahoma. |
| Late 1800s | U.S.A. | E.W. Hilgard promoted the use of hydrochloric acid as an extractant for determining fertility status of soils. |
| 1909 | Germany | E.A. Mitscherlich developed his equation relating growth to the supply of plant nutrients. |
| Early 1900s | U.S.A. | C.G. Hopkins promoted the importance of monitoring changes in soil fertility status to prevent decreases in productivity as a result of nutrient depletion. |
| 1940s and 50s | U.S.A. | Introduction of new crop varieties and hybrids and increases in the availability and use of fertilizers spurred interest in soil testing as a management tool. |
| 1960’s to present | U.S.A. | Evolution of soil testing continues on all fronts as technological advances allow improvements in the areas of analysis, correlation, calibration and interpretation. |
Soil testing in Oklahoma first became popular in the 1950s. Soil testing for farmers primarily was performed by county extension agents (now called educators) who operated small laboratories out of their county offices. Samples periodically were analyzed by researchers at the OSU campus to verify their accuracy. In the 1960s, Dr. Billy Tucker, an extension soil fertility specialist, and Dr. Lester Reed, a soil chemist, helped analyze approximately 200 to 300 samples per year for the county agents.
After several years, Dr. Tucker realized advances in research and technology were causing the county soil testing laboratories to become outdated. In order to maintain a quality soil testing/soil fertility program at OSU, a centralized state soil testing laboratory was needed that used standardized methods and interpretations based on statewide research.
The task was easier said than done. Much resistance was met from the county agents, who took pride in their soil testing skills and also saw their laboratories as a means of making contacts with farmers and generating extra income for other Extension programs. After much public and private debate, Dr. Tucker finally convinced the director of Extension and most county agents to support the establishment of a centralized soil testing laboratory on the OSU campus. Since that time (1969), sample activity at the OSU laboratory has grown to approximately 28,000 soil samples per year.
Value of Soil Testing
Soil tests are designed to estimate plant-available fractions of selected nutrients, that is, the portion of a nutrient present in the soil that a plant can take up. Soil fertility tests do not measure total amounts of nutrients in the soil because not all chemical forms of the nutrient can be used by the plant. As a soil test level increases for a particular nutrient, the ability of the soil to supply that nutrient also increases and less fertilizer needs to be added to adequately supply food for the plant.
Much field and laboratory research must be conducted to accurately interpret soil tests so proper amounts of fertilizer are recommended for application. This process is called calibration. During the calibration process, a relationship is established between the soil test value and the amount of fertilizer needed by the plant. Soil tests are calibrated by establishing fertilizer rate experiments on soils with different soil test levels to determine the best fertilizer rate for each level. Once a number of fertilizer experiments have been conducted, the data can be summarized and fertilizer recommendation guides can be developed. Agricultural Experiment Stations provide this information.
Soil Sampling
Producers and fertilizer dealers must remember a good soil sample is obtained by sampling a uniform field area. Avoid sampling “odd-ball” areas. Sample each field separately, as well as dissimilar soil types within the same field. A core or slice from the surface to a depth of 6 inch or plow layer should be taken from 15 to 20 locations in the field and composited into one representative sample to be tested.
Noncultivated fields should be sampled to a depth of six inches, again because this is the effective depth of most treatments and the depth of most root activity. Nutrients from fertilizer, animal manure and lime can be accumulated on the surface if they are surface applied without incorporation. A set of samples from the top two inches will help identify stratification of nutrients and is especially important for pH determination for no-till fields. If nutrient loss in runoff is the main concern, the two-inch sample is better than a six-inch sample because only the surface inch or two is in direct contact with surface runoff.
Special attentions should to be paid when sampling fields where fertilizers are banded. See Fact Sheet PSS-2207 How to Get a Good Soil Sample for details.
Subsoil samples for nitrates are valuable for estimating fertilizer nitrogen carryover. The nitrogen fertilizer rate easily is adjusted to take advantage of “leftover” nitrate. The subsoil test should be taken from 6 to 18 inches. Sample depth should be indicated when submitting subsoil samples for the nitrate test. Subsoil sample analysis can help provide a more reliable estimate of other nutrients that are mobile in the soil, such as boron, sulfur, and chloride.
Soil samples may be submitted to your county OSU Extension office. They will send the samples to the Soil, Water and Forage Analytical Laboratory for testing and then send the results back to you with fertilizer recommendations. Soil samples are analyzed routinely for pH, nitrate nitrogen, plant available phosphorus and potassium, while calcium, magnesium, sulfur (secondary nutrients), zinc, iron and boron (micronutrients) are tested on request. The subsoil is analyzed only for nitrate unless otherwise requested. A number of other tests also are available through the lab.
Laboratory Soil Tests
A brief description of laboratory tests currently used at the OSU lab follows.
pH
This test measures the active soil acidity or alkalinity. Soils with a pH of 7.0 are neutral soil; pH less than 7 is acid and soil pH values higher than 7.0 are alkaline. Under normal conditions, most plants grow well when soil pH is in the range of 6.0 to 7.5. An application of lime should be considered for most non-legume crops when soil pH is 5.5 or less. Legumes usually grow best when the pH is 6.0 or higher.
Buffer Index
When soil pH is less than 6.3, a buffer index reading is obtained. This value estimates the amount of lime required to correct soil acidity. The buffer index value is not a standard pH reading and means nothing without a calibration table that relates it to the amount of lime to apply. The lower the buffer index, the higher the lime requirement (See Chapter 3 for more details about pH and liming).
Nitrate
The nitrate soil test measures the actual amount of nitrate-nitrogen in the soil available to plants. The nitrogen fertilizer requirement can be determined by subtracting the pounds of nitrate-nitrogen in the soil from the total nitrogen requirement for a selected yield goal.
Phosphorus
The phosphorus soil test estimates the amount of available soil phosphorus. The actual amount cannot be measured because of chemical reactions occurring in the soil. The estimated availability is reported as soil test index and a percent sufficiency in the soil. A soil test with 40 percent sufficiency means 40 percent of plant phosphorus needs will be supplied by the soil. The remainder must be provided by adding fertilizer to reach the 100 percent potential yields. If no phosphorus is added, the yield will only be 40 percent of its potential. Much field calibration work must be done to correctly interpret this type of test. The Mehlich-3 procedure is used for extraction of soil phosphorus and potassium in Oklahoma. Other labs may use different procedures. Oklahoma calibration may not be appropriate if soils are tested with a different method.
Potassium
Like phosphorus soil tests, potassium tests estimate availability and indicate a certain percent sufficiency.
Calcium and Magnesium
These two elements and potassium are referred to as exchangeable cations and are found on the cation exchange sites of the soil. The soil tests measure the exchangeable portion of the cations. Oklahoma research has found that calcium and magnesium additions can increase yields when individual tests are low. Percent of base saturation or ratios of calcium/magnesium, potassium/magnesium, calcium/potassium or calcium/magnesium/potassium have not been useful in depicting deficiencies on most Oklahoma soils.
Sulfur
The sulfur soil test measures the amount of available sulfate-sulfur. The amount found in the soil test can be subtracted from crop requirements based upon a yield goal similar to the approach used for nitrogen. Unlike nitrogen, most soils contain adequate available sulfur for most crops. Additionally, annual sulfur contributions from rainfall are high enough to meet the needs of a 60-bushel wheat crop.
Zinc, Iron and Boron
Availability of these trace or micronutrient elements can be estimated from soil tests. Trace element deficiencies occur only on certain soils and with certain crops. Knowledge of crop needs and soil deficiencies will help determine when trace element tests need to be run.
Soil Test Interpretations
After soil samples have been tested, the results need to be examined to see if they identify nutrient deficiencies in any of the fields. This step is called interpreting the test results. Interpretation can only be done reliably if the soil test has been calibrated by field research. Usually calibration research is on-going at land-grant universities, such as OSU, and has its best application for soils in that state. The calibration should identify the deficiency and estimate its severity.
OSU interpretations are based on research calibration tables published in Extension Fact Sheet PSS-2225. The same calibration tables are included here as a reference (Tables 4.3 to 4.10). The tables in PSS-2225 are updated periodically as determined by current research results.
Primary Nutrient Interpretations
Soil test interpretations for nitrogen, phosphorus and potassium are presented in Tables 4.3-4.6. Fertilizer requirements for common Oklahoma crops and forages can be determined from these tables. Nitrogen requirements are based on yield goal, while phosphorus and potassium requirements are based on soil test values and their corresponding sufficiency levels.
Interpretations of soil test reports obtained from OSU are automatically generated by computer using data from these calibration tables. An example report is shown in Figure 4.1. The report lists the name and address of the sender at the top and presents the sample identification numbers and soil test results in designated boxes below. The soil test interpretation is printed in an area underneath the test results. If no cropping information is provided with a soil sample, then no computer interpretation is generated and fertilizer requirements must be determined by use of the calibration tables in Fact Sheet PSS-2225 or an interactive program on the lab’s website. A yield goal also is needed to make nitrogen recommendation except for lawn and gardens.
Figure 4.1: Example soil test report from the OSU Soil, Water and Forage Analytical Laboratory
In the example report, wheat was selected as the crop and 50 bushels per acre was selected as the yield goal. Both selections are listed at the beginning of the interpretation. The pH of the sample was 6.5 which is satisfactory for wheat, therefore no lime was required.
The nitrate test for this sample showed 20 pounds nitrogen per acre in the soil. According to the calibration tables (Table 4.3), 50 bu/acre of wheat requires 100 pounds per acre of nitrogen, Subtracting 20 from 100 results in a deficiency of 80 pounds nitrogen per acre which must be supplied using nitrogen fertilizer.
The phosphorus test index for this sample was 10. The calibration table for wheat (Table 4.3) shows that a phosphorus index of 10 corresponds to a sufficiency level of 45 percent. The corresponding P2O5 fertilizer requirement to offset this insufficiency is shown on the report or can be read directly from the calibration table as 60 pounds per acre. This rate of P2O5 must be applied annually to prevent phosphorus deficiency until another soil test is performed.
The potassium test index for this sample was 100. This value is not listed in the potassium calibration table for wheat, so the fertilizer requirement must be estimated using the requirements recommended for the index values, 75 and 125 (Table 4.3). Since 100 is halfway between 75 and 125, the potassium index of 100 corresponds to a sufficiency level of approximately 75 percent (halfway between 70 and 80) and a K2O requirement of approximately 45 pounds per acre (halfway between 50 and 40). The computer calculated this value and listed the potassium fertilizer requirement as a “75 percent sufficiency, 45 pounds per acre K2O.” This rate of K2O, like P2O5 , must be applied annually to prevent potassium deficiency until another soil test is performed.
Table 4.3 Primary nutrient soil test calibration tables for small grains and row crops
| Small Grain | Grain Sorghum | Corn | Cotton | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yield Goal Bu/A | N | Yield Goal | N | Yield Goal | N | Yield Goal | N | |||
| Wheat | Barley | Oats | lb/a | lb/a | lb/a | bu/a | lbs/a | bales/a | lb/a | |
| 15 | 20 | 25 | 30 | 2,000 | 30 | 40 | 40 | 1.0 | 5.0 | |
| 20 | 25 | 35 | 40 | 2,500 | 40 | 50 | 50 | 1.5 | 75 | |
| 30 | 35 | 55 | 60 | 3,000 | 50 | 60 | 60 | 2.0 | 100 | |
| 40 | 50 | 70 | 80 | 4,000 | 70 | 85 | 85 | 2.5 | 125 | |
| 50 | 60 | 90 | 100 | 4,500 | 85 | 100 | 110 | 3.0 | 150 | |
| 60 | 75 | 105 | 125 | 5000 | 100 | 120 | 130 | 3.5 | 175 | |
| 70 | 90 | 125 | 155 | 7000 | 160 | 160 | 190 | >3.5 | 175 | |
| 80 | 100 | 140 | 185 | 8000 | 195 | 180 | 215 | - | - | |
| 100 | 125 | 175 | 240 | 9000 | 230 | 200 | 240 | - | - |
Table 4.3 Continued
| P Soil Test Index |
Small Grain | Grain Sorghum | Corn | Cotton | |||||
|---|---|---|---|---|---|---|---|---|---|
| Percent Sufficiency |
P2O5 lb/a |
Percent Sufficiency |
P2O5 lb/a |
Percent Sufficiency |
P2O5 lb/a |
Percent Sufficiency |
P2O5 bales/a |
||
| 0 | 25 | 80 | 40 | 60 | 30 | 80 | 55 | 75 | |
| 10 | 45 | 60 | 60 | 50 | 60 | 60 | 70 | 60 | |
| 20 | 80 | 40 | 80 | 40 | 80 | 40 | 85 | 45 | |
| 40 | 90 | 20 | 95 | 20 | 95 | 20 | 95 | 30 | |
| 65+ | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
Table 4.3 Continued
| K Soil Test Index |
Small Grain | Grain Sorghum | Corn | Cotton | |||||
|---|---|---|---|---|---|---|---|---|---|
| Percent Sufficiency |
K2O | Percent Sufficiency |
K2O lb/a |
Percent Sufficiency |
K2O lb/a |
Percent Sufficiency |
K2O bales/s |
||
| 0 | 50 | 60 | 40 | 100 | 40 | 120 | 40 | 110 | |
| 75 | 70 | 50 | 65 | 75 | 60 | 80 | 60 | 80 | |
| 125 | 80 | 40 | 80 | 50 | 75 | 60 | 75 | 60 | |
| 200 | 95 | 20 | 95 | 30 | 90 | 40 | 90 | 40 | |
| 250+ | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
Table 4.4 Primary nutrient soil test calibration tables for selected grasses and silage
| Cool Season Grasses (fescue, orchard, rye) |
Weeping Lovegrass | Bluestem | Bermudagrass | Forage Sorghum, Corn-ensilage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield Goal Tons/A |
N lbs/A |
Yield Goal Tons/a |
N lbs/a |
Yield Goal Tons/a |
N lbs/a |
Yield Goal Tons/a |
N lbs/Aa |
Yield Goal Ensilage |
Tons/a Hay |
N lbs/a |
|
| 1 | 60 | 1 | 35 | 1 | 35 | 1 | 50 | - | 1.0 | 18 | |
| 2 | 120 | 2 | 70 | 2 | 70 | 2 | 100 | 2 | 2.5 | 45 | |
| 3 | 180 | 3 | 110 | 3 | 110 | 3 | 150 | 10 | 5.0 | 90 | |
| 4 | 240 | 4 | 160 | 4 | 150 | 4 | 200 | 15 | 7.5 | 135 | |
| 5 | 300 | 5 | 220 | 5 | 200 | 5 | 260 | 20 | 10.0 | 185 | |
| - | - | - | - | - | 6 | 320 | 25 | 12.5 | 240 | - | |
| - | - | - | - | - | 7 | 400 | 30 | 15.0 | 300 | - |
Table 4.4 Continued
| P Soil Test Index |
Cool Season Grasses (fescue,orchard,rye) |
Weeping Lovegrass | Bluestem | Bermudagrass | Forage Sorghum, Corn-Ensilage |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent Sufficiency | P2O5 lbs/a |
Percent Sufficiency | P2O5 lbs/a |
Percent Sufficiency | P2O5 lbs/a |
Percent Sufficiency | P2O5 lbs/a |
Percent Sufficiency | P2O5 lbs/a |
||
| 0 | 30 | 80 | 50 | 60 | 50 | 60 | 50 | 75 | 30 | 100 | |
| 10 | 50 | 60 | 70 | 40 | 70 | 40 | 65 | 60 | 60 | 75 | |
| 20 | 70 | 40 | 85 | 30 | 85 | 30 | 80 | 40 | 80 | 45 | |
| 40 | 95 | 30 | 95 | 20 | 95 | 20 | 95 | 20 | 95 | 25 | |
| 65+ | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
Table 4.4 Continued
| K Soil Test Index |
Cool Season Grasses | Weeping Lovegrass | Bluestem | Bermudagrass | Forage Sorghum, Corn-Ensilage |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent Sufficiency | K2O lbs/a |
Percent Sufficiency | K2O lbs/a |
Percent Sufficiency | K2O lbs/a |
Percent Sufficiency | K2O lbs/a |
Percent Sufficiency | K2O lbs/a |
||
| 0 | 60 | 70 | 40 | 80 | 40 | 80 | 50 | 140 | 40 | 180 | |
| 75 | 70 | 60 | 65 | 60 | 60 | 60 | 65 | 80 | 60 | 130 | |
| 125 | 80 | 50 | 80 | 40 | 80 | 40 | 80 | 50 | 75 | 90 | |
| 200 | 95 | 30 | 95 | 20 | 95 | 20 | 95 | 30 | 90 | 60 | |
| 250+ | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
Table 4.5 Primary nutrient soil test calibration tables for selected forages.
| Small Grains for Grazing | Legumes In Pasture | New Seeding of Introduced Grasses | Virgin Native Hay Meadows | |||
|---|---|---|---|---|---|---|
| Yield Goal tons/a |
N lbs/a |
Legumes will produce nitrogen for their growth. Very little nitrogen remains for the grasses after legume growth stops unless the legume growth is not harvested but is allowed to decay. | 40 lb of nitrogen needed to establish a grass. Refer to other table for N requirement for production. | Yield Goal tons/a |
N lbs/a |
|
| 1/2 | 30 | 1.0 | 0 | |||
| 1 | 60 | 1.5 | 50 | |||
| 11/2 | 90 | 2.0 | 100 | |||
| 2 | 120 | |||||
| 21/2 | 150 | |||||
| 3 | 180 |
Table 4.5 Continued
| P Soil Test Index |
Small Grains For Grazing | Legumes in Pasture | New Seeding of Introduced Grasses | Virgin Native Hay Meadows | |||||
|---|---|---|---|---|---|---|---|---|---|
| Percent Sufficiency | P2O5 | Percent Sufficiency | P2O5 | Percent Sufficiency | P2O5 | Percent Sufficiency | P2O5 | ||
| 0 | 25 | 80 | 50 | 75 | 30 | 80 | 50 | 40 | |
| 10 | 45 | 60 | 65 | 60 | 50 | 60 | 80 | 20 | |
| 20 | 80 | 40 | 80 | 40 | 70 | 40 | 95 | 0 | |
| 40 | 90 | 20 | 95 | 20 | 95 | 20 | 100 | 0 | |
| 65+ | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
Table 4.5 Continued
| K Soil Test Index |
Small Grains for Grazing | Legumes in Pasture | New Seeding of Introduced Grasses | Virgin Native Hay Meadows | |||||
|---|---|---|---|---|---|---|---|---|---|
| Percent Sufficiency | K2O5 | Percent Sufficiency | K2O5 | Percent Sufficiency | K2O5 | Percent Sufficiency | K2O5 | ||
| 0 | 50 | 60 | 50 | 80 | 50 | 80 | 40 | 40 | |
| 75 | 70 | 50 | 65 | 60 | 65 | 60 | 70 | 30 | |
| 125 | 80 | 40 | 80 | 40 | 80 | 40 | 85 | 20 | |
| 200 | 95 | 20 | 95 | 20 | 95 | 20 | 95 | 0 | |
| 250+ | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
Table 4.6 Primary nutrient soil test calibration tables for legumes.
| Alfalfa | Peanuts | Soybeans | Mungbeans, Cowpeas, Guar | |
|---|---|---|---|---|
| 10-20lb/A for establishment. None Needed for maintenance. | 10-20lb N/A with P&K | 10-20lb N/A with P&K Inoculate seed. | 10-20lbs N/A with P&K Inoculate seed. |
Table 4.6 Continued
| P Soil Test Index |
Alfalfa | Peanuts | Soybeans | Mungbeans, Cowpeas, Buar | |||||
|---|---|---|---|---|---|---|---|---|---|
| Percent Sufficiency | P2O5 | Percent Sufficiency | P2O5 | Percent Sufficiency | P2O5 | Percent Sufficiency | P2O5 | ||
| 0 | 20 | 200 | 40 | 80 | 40 | 70 | 40 | 70 | |
| 10 | 50 | 150 | 60 | 60 | 60 | 50 | 60 | 50 | |
| 20 | 70 | 100 | 80 | 40 | 80 | 30 | 80 | 30 | |
| 40 | 90 | 60 | 95 | 20 | 95 | 20 | 95 | 20 | |
| 65+ | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
Table 4.6 Continued
| K Soil Test Index |
Alfalfa | Peanuts | Soybeans | Mungbeans, Cowpeas, Buar | |||||
|---|---|---|---|---|---|---|---|---|---|
| Percent Sufficiency | P2O5 | Percent Sufficiency | P2O5 | Percent Sufficiency | P2O5 | Percent Sufficiency | P2O5 | ||
| 0 | 20 | 280 | 40 | 80 | 40 | 100 | 50 | 80 | |
| 75 | 50 | 210 | 60 | 60 | 60 | 70 | 60 | 60 | |
Secondary and Micro-nutrient Interpretations
Calcium
Calcium deficiency has not been observed in any crop in Oklahoma. Gypsum is sometimes applied over the pegging zone of peanuts during early bloom stage to improve quality. Appropriate rates are listed in Table 4.7.
Table 4.7. Calcium soil test interpretation for peanuts.
|
Ca Soil Test Index (pounds per acre) |
Gypsum Needed (pounds per acre) |
|---|---|
| 0 | 750 |
| 150 | 500 |
| 300 | 400 |
| 450 | 300 |
| 600 | 200 |
| >750 | 0 |
Magnesium
Magnesium deficiencies are indicated by soil test index values less than 100 pounds per acre. Deficiencies can be corrected by applying 30-40 lbs of magnesium per acre from fertilizer source or by using dolomite limestone if lime is needed.
Sulfur
Sulfur is a mobile nutrient in the soil, therefore, plant requirements are based on yield goal similar to that for nitrogen, Sulfur requirements for non-legumes are calculated by dividing the nitrogen requirement by 10. The available sulfur measured by the sulfur soil test for both the surface and subsoil is subtracted from the sulfur requirement to determine the fertilizer rate. The rate may also be reduced by an additional 6 lb/acre due to sulfur supplied through rainfall and other incidental additions such as nitrogen, phosphorus and potassium fertilizer impurities. Following is an example of sulfur interpretation for Bermudagrass:
Crop: Bermudagrass Yield goal: 6 tons/acre
N requirement (Table 4.4) = 320 pounds per acre
Sulfur requirement = nitrogen req./20 = 320/10 = 32 pounds per acre
Sulfur soil test values: surface = 5 pounds per acre
subsoil = 12 pounds per acre
total = 17 pounds per acre
Incidental sulfur additions: 6 pounds per acre
Sulfur fertilizer rate = 32-17-6 = 9 pounds sulfur per acre
Table 4.8. Sulfur requirements for legumes
| Alfalfa | Peanuts | Soybeans | Mungbeans | Cowpeas | Guar | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Yield Goal tons/acre |
S lbs/acre |
Yield Goal tons/acre |
S lbs/acre |
Yield Goal tons/acre |
S lbs/acre |
Yield Goal tons/acre |
S lbs/acre |
Yield Goal tons/acre |
S lbs/acre |
Yield Goal tons/acre |
S lbs/acre |
| 2 | 12 | 6 | 4 | 10 | 6 | 5 | 3 | 5 | 3 | 6 | 4 |
| 4 | 22 | 12 | 6 | 20 | 12 | 10 | 6 | 10 | 5 | 12 | 6 |
| 6 | 34 | 18 | 10 | 30 | 18 | 15 | 9 | 15 | 8 | 18 | 10 |
| 8 | 44 | 24 | 14 | 40 | 24 | 20 | 12 | 20 | 11 | 24 | 14 |
| 10 | 56 | 30 | 18 | 50 | 30 | ||||||
| 36 | 22 | 60 | 36 | ||||||||
A similar calculation is used to determine the sulfur fertilizer rate for legumes, with the exception that the sulfur requirement is obtained from Table 4.8 rather than dividing the nitrogen requirement by 10.
Zinc
The soil test interpretation for zinc is presented in Table 4.9. Zinc soil test values less than 0.30 parts per million are considered deficient for all crops except small grains, cool season grasses (fescue, orchardgrass and ryegrass) and new seedings of introduced grasses. The recommended rates are enough to correct a deficiency for several years. Fertilizer applications should not be repeated until a new soil test is taken. Some producers may wish to apply 2 pounds of zinc per year until the total recommended amount is reached.
Iron
Iron soil test values less than 2.0 parts per million are considered low and may cause iron chlorosis in crops which are moderately sensitive such as wheat, soybeans and peanuts. Soil test values in the medium range, 2.0 to 4.5 parts per million, may cause chlorosis in sensitive crops such as sorghum and sudan. Levels above 4.5 parts per million are usually adequate for all crops. Crop sensitivity is increased when soil pH increases above 8.2 and soil test manganese levels are high (above 50 parts per million). Foliar application of a 3 percent ferrous sulfate (or ammonium ferrous sulfate) solution is effective for correction. Severe chlorosis may require several applications. Effective control can be obtained by applying 2 pounds of iron per acre in chelated form or 8 pounds of ferrous sulfate per acre with ammonium polyphosphate solution in a band near the seed. It is important to apply the polyphosphate and ferrous sulfate solutions in the same band.
Boron
Boron deficiency in Oklahoma is of concern only in legumes, particularly alfalfa and peanuts. The soil test interpretation for boron is presented in Table 4.10.
Table 4.10. Boron soil test interpretation.
|
Soil test B (ppm) |
Boron rate (lb/a) | |
|---|---|---|
| Peanuts | Alfalfa | |
| 0.0-0.25 | 1 | 2 |
| 0.25-0.50 | 0.5 | 1 |
| 0.50 | 0 | 0 |
Nutrient Deficiency Symptoms
Identifying nutrient deficiency symptoms is sometimes helpful in assessing fertility problems that need correction. Plant analysis may be used to confirm deficiency symptoms or monitor fertilizer effectiveness.
Recognizing nutrient deficiency symptoms and obtaining plant analysis are good approaches for identifying fertility problems but are not suitable parameters for making fertilizer recommendations. These two approaches are useful for identifying problem areas that need to be soil tested to measure the severity of the deficiency and the fertilizer requirements.
Plants deficient in one or more essential nutrients become “sick” and exhibit different leaf colors and growth disorders that are indicative of the deficiency. With practice one can identify symptoms and make suggestions for remedies.
The problem for most is identifying the deficiency symptom correctly. The key presented in Table 4.11 should be helpful. A more complete description of deficiency symptoms that may be observed in Oklahoma follows.
Nitrogen
Nitrogen is the most universally deficient nutrient in nonlegumes. A deficient field will possess a light green appearance. When nitrogen deficiency occurs later in plant growth, yellowing begins at the leaf tip and follows up the leaf midrib in a V-shaped pattern of the oldest leaves. Eventually, the entire lower leaf of plants, e.g., corn. will turn yellow and then brown (necrosis or death of tissue). As this happens, the second and third leaf will show chlorosis of the tip and midrib tissue as nitrogen is translocated to new leaves. A few days after the leaf tissue turns yellow, it dies and dries up.
Table 4.11. Key to nutrient deficiency symptoms.
| Symptom | Deficient Nutrient |
|---|---|
| A. Color change in lower (older) leaves. | |
| 1. Plants light green - lower leaves yellow from tip along midrib towards base. | Nitrogen |
| 2. Plants dark green, some purple coloring on base of stem - leaves and plants small. | Phosphorus |
| 3. Brown discoloration and scorching along outer margins of lower leaves. | Potassium |
| 4. Lower leaves have yellow discoloration between veins - reddish-purple cast from edge inward in some plants. | Magnesium |
| B. Color changes in upper (newer) leaves. | |
| 1. Terminal bud dies. | |
| a. Emergence of primary leaves delayed - terminal buds deteriorate. | Calcium |
| b. Leaves near growing point yellowed - growth buds appear as white or light brown dead tissue. | Boron |
| 2. Terminal bud remains alive. | |
| a. Leaves including veins turn pale green to yellow - young leaves first. | Sulfur |
| b. Leaves yellow to almost white - interveinal chlorosis to tip of leaf. | Iron |
| c. Shortened internodes – pale yellow or bronze coloration between leaf margin and midrib. | Zinc |
| d. Leaves yellowish-gray or reddish-gray with green veins. | Manganese |
| e. Young leaves uniformly pale yellow - may wilt and wither without chlorosis. | Copper |
| f. Wilting of upper leaves - followed by chlorosis. | Chlorine |
| g. Young leaves wilt and die along the margins. | Molybdenum |
Phosphorus
Mild phosphorus deficiencies are characterized by stunted growth and an abnormally green appearance. In the advanced stages, phosphorus deficiencies cause purpling of the leaves. As in the case of nitrogen, the symptoms start with the older leaves and progress upward toward the younger leaves. Eventually leaf tips die and turn brown. Phosphorus deficiencies are more pronounced in young plants. Absorption of phosphorus by plants is slowed by cool soil. Often phosphorus deficiencies dissipate as the soil warms if sufficient phosphorus is present in available forms.
Whenever sorghum, corn and cereals are damaged by certain insecticides, a purple pigmentation develops in the leaves. This leaf discoloration should not be confused with phosphate deficiency.
Potassium
Potassium deficiency causes shorter plants, weaker stems or stalks and a general loss of green color. Severe deficiencies produce a discoloration of the leaf tip and edges. In sorghum, corn, cotton and other large-leafed plants, the discoloration on the leaf edges is continuous. Potassium deficiency of grains and legumes is a general yellow mottling as well as numerous brown specks which occur at leaf tips, around margins and between the veins. As symptoms progress, the yellow mottled spots on leaf edges die and finally the dead tissue sloughs off giving leaves an extremely ragged appearance. The dying of the lower leaf is referred to as firing. The condition known as firing is usually caused by potassium deficiency but other conditions such as dry and hot weather can also bring about dead tissue in the leaves and can be confused with potassium and nitrogen deficiency.
Potassium deficiency symptoms are rarely seen on peanuts. Fruit crops and many ornamental plants are highly susceptible to potassium deficiencies, and broad-leafed trees and ornamental plants readily show potassium deficiencies. Potassium deficiency in Bermudagrass increases its susceptibility to winter kill.
Sulfur
Sulfur deficiencies usually result in stunted growth, delayed maturity and a general yellowing of the foliage. Since it is easy to mistake sulfur deficiency for nitrogen deficiency, one must know the nitrogen status before diagnosing a sulfur deficiency. Sulfur deficiency is more pronounced on young leaves.
In many sulfur-deficient plants, the veins remain green even though the tissue between the veins becomes chlorotic giving the leaf a mottled appearance. These mottled leaves resemble iron and zinc deficiencies.
Magnesium
Magnesium deficiency occurs first on the lower leaves as a general yellowing. Eventually the areas between the veins of the leaves become light yellow giving rise to a striping on grass-type plants and mottling on broadleaf plants. In some plants, like soybeans, rusty specks and necrotic blotches may appear between the veins and around the edges of the newest leaflets. In cotton, magnesium deficient plants are purplish-red with green veins. Late in the season it is difficult to distinguish between magnesium deficiency and normal maturity in cotton, which produces a purplish-red leaf.
Zinc
Zinc deficiency symptoms usually are seen during the plant seedling stage. It is characterized by a broad band of bleached tissue on each side of the midrib beginning at the base of the leaf. The midribs and leaf edges remain green. On broadleaf plants, a general bronzing may occur with a pronounced interveinal chlorosis. The leaves become thick and brittle, and their margins are cupped upward. In grain sorghum, heads from severely zinc-deficient plants are blasted. Most crops fail to develop normal internode length resulting in severe stunting and an appearance of all leaves coming from the same node.
Iron
Iron deficiency can be detected by yellowing between the veins with the veins remaining green. This gives a striping appearance. In contrast to zinc deficiency, the stripes are much narrower and extend the full length of the leaf.
Iron is not mobile within the plant, therefore, a deficiency first is observed on the younger (top) leaves with the older part of the plant remaining green. In severe cases the terminal portion of the plant turns white and eventually dies.
Boron
Boron deficiencies develop first on the youngest growth. The upper internodes are shortened and plants develop a rosette appearance. Upper leaves near the growing point turn yellow and in some legumes are reddened. The lower leaves remain green and healthy. In severe cases the terminal leaves become white.
In cotton, boron deficiency is described as having thick and leathery older leaves. Leaf petioles often are twisted with small ruptures appearing over their surfaces. A constriction near the base of the petiole may occur giving a ringed condition. Severe boron deficiency in cotton results in half opened bolls and plants that are hard to defoliate.
Boron deficient peanut plants possess the typical yellowing and rosetting, but even before the symptoms are noted on the vines, the nuts may have internal damage. The center of the nut will be somewhat hollow and discolored. Nuts with hollow heart are severely downgraded upon marketing.
Other Deficiency Symptoms
Other nutrients exhibit characteristic deficiency symptoms, but the expected occurrences of these deficiencies in Oklahoma are rather remote.
Assistance should be obtained from a qualified person and/or plant analysis and soil tests to confirm the symptom, since chlorosis or yellowing and brown spots can result from factors other than nutrient deficiency. Herbicide damage and excess amounts of elements can cause similar visual symptoms. The deficiency must be confirmed before attempting to correct it. There are a number of “apps” available to show nutrient deficiency symptoms, such as “Yara CheckIT” and others to be downloaded to your smart phone.
Sometimes the knowledge of environmental conditions is useful in diagnosing the nutrient problem. These conditions should be checked:
Root zone - The soil should be granular and permeable so roots may expand and feed extensively. Crops normally develop a root system to a depth of 3 to 5 feet from which they extract water and nutrients. A shallow or compacted soil does not offer this root a favorable feeding zone.
Temperature - Cool soil temperatures reduce organic matter decomposition and the amount of nitrogen and other nutrients being released. Solubility of elements is lower in cool temperatures, thus creating more deficiencies.
Soil pH - The availability of some plant nutrients is greatly affected by soil pH. Molybdenum availability is reduced by acid soil conditions, while iron, manganese, boron, copper, and zinc availabilities are increased by soil acidity. Nitrogen and phosphorus availabilities are highest between a pH of 5.5 and 7.2. Aluminum toxicity may occur in very acidic soils, which also result in a purple leaves.
Insects - Insect damage may look like deficiency symptoms. Roots should be examined for insect damage that may project itself as a nutrient deficiency.
Diseases - Close study will reveal differences between plant diseases and nutrient deficiency symptoms. The organisms can usually be found upon close examination.
Moisture conditions - Dry soil conditions may create deficiencies. However, nutrient deficiencies during drought must be correctly identified and not attributed to the drought. Crop “firing” attributed to the drought may actually be nitrogen or potassium deficiency.
Soil salinity problems - In some areas of Oklahoma soluble salts and alkali are problems. These areas usually cover only a portion of the field. The salty areas usually occur where a high water table exists, saltwater well contamination has occurred or poor quality water has been used for irrigation.
Nutrient deficiency - Symptoms indicate severe starvation problems but have the shortcoming of not indicating slight to moderate starvation. Many crops exhibit yield reductions from a lack of nutrition before actually showing visual signs of a deficiency. Hidden hunger is the term used to describe this phenomenon. Hidden hunger may reduce yields and quality of crops without the plants showing deficiency symptoms.
Plant Analysis
The term plant analysis means the chemical analysis of plant tissue to determine the concentration of essential plant nutrients, excluding carbon, hydrogen and oxygen. The level of nutrients in the plant tissue is compared to established sufficiency levels to determine possible deficiencies and hidden hunger. In some cases poor-growth plant tissue may be compared to adjacent good-growth plant tissue to draw conclusions about the problem area.
Plant analysis can be used to measure the level of plant nutrients that are difficult to test by soil testing procedures, such as molybdenum. It is a good tool for researchers to use when evaluating fertilizer sources or fertilizer placement and when confirming nutrient deficiency symptoms. Plant analysis cannot be used to make fertilizer recommendations because the soil pH and soil nutrient level must be known. It can be used to adjust the fertilizer recommendation once the soil level is known. The same factors that interfere with identifying nutrient deficiency symptoms must be considered when interpreting plant analysis.
A proper plant sample must be taken for plant analysis to be reliably interpreted. Sufficiency levels have been established for certain plant parts as shown in Table 4.12. Sufficiency ranges for more plants can be found in Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States (http://www.clemson.edu/sera6/scsb394.pdf).
Table 4.12. Sufficiency levels of plant nutrients for several crops at recommended stages of growth shown in Table 4.13.
| Element | Sufficiency Levels | ||||||
|---|---|---|---|---|---|---|---|
| Corn |
Grain Sorghum |
Soybeans |
Small Grains |
Peanuts | Alfalfa | Bermudagrass | |
| N, % | 2.7-3.5 | 3.3-4.0 | 4.2-5.5 | 1.7-3.0 | 3.5-4.5 | 4.5-5.0 | 2.5-3.0 |
| P,% | .25-.40 | .20-.35 | .26-.50 | .20-.50 | .20-3.5 | .26-.70 | .26-.32 |
| K,% | 1.7-2.5 | 1.4-2.5 | 1.7-2.5 | 1.5-3.0 | 1.7-3.0 | 2.0-3.5 | 1.8-2.1 |
| Ca, % | .21-1.0 | .30-.60 | .36-2.0 | .20-.50 | 1.25-1.75 | .50-3.0 | |
| Mg, % | .21-.60 | .20-.50 | .26-1.0 | .15-.50 | .30-.80 | .30-1.0 | |
| S, % | .20-.30 | .26-.50 | .15-.20 | ||||
| B, ppm | 4-25 | 1-10 | 21-55 | 5-10 | 20-50 | 30-80 | |
| Cu, ppm | 2-6 | 2-7 | 10-30 | 5-25 | 10-50 | 7-30 | |
| Fe, ppm | 21-25 | 65-100 | 51-350 | 50-150 | 100-350 | ||
| Mn, ppm | 20-150 | 8-190 | 21-100 | 25-100 | 100-350 | 31-100 | |
| Zn, ppm | 20-70 | 15-30 | 21-50 | 15-70 | 20-50 | 21-70 |
Select plant tissue so it represents the field as much as possible. Take the composite sample by sampling the number of plants shown in Table 4.13. The same procedure should be used when sampling abnormal growth areas in a field (i.e. take the required number of plants throughout the trouble spot and select an equal-size area of normal plants to sample for comparative purposes).
Keep in mind that disease- or insect-infected plants, drought-stricken plants and frost-damaged plants should not be sampled.
Allow samples to partially dry before mailing. Send samples in paper bags or envelopes, not in plastic bags. Damp or wet plant tissue will deteriorate if mailed in plastic or air-tight containers. Do not send soil or roots in the same container. Soil contaminates the plant tissue and makes it difficult to clean at the laboratory.
It is a good idea to take a soil sample in the same vicinity as the plant sample. Soil tests may help interpret the plant analysis results. Plant tissue sufficiency levels for several crops are presented in Table 4.12. Whenever nutrient levels in the plants fall below the sufficiency range, a deficiency is expected. The lower the concentration is below the sufficiency range, the greater the nutrient deficiency.
Some laboratories and researchers have tried to use ratios between 2 or more elements for interpretation. At the present time, the N/S ratio appears to be a good method for diagnosing sulfur deficiency. Sulfur is sufficient when the ratio is 15:1 or less and deficient when the ratio is greater than 20:1. Other combinations or ratios have not shown any benefit over the sufficiency levels shown in Table 4.12.
Remember to use plant analysis along with other data, including soil tests and plant samples from a normal area in the same field. Interpretation must be logical. Be suspicious of far-fetched diagnosis. Growers have frequently been disappointed by applying some otherwise illogical nutrient to their soil and obtaining no benefit. The OSU Soil, Water and Forage Analytical Laboratory conducts plant analysis and list sufficiency ranges similar to those in Table 4.12.
Table 4.13. Guide to plant sampling for tissue analysis.
| Crop | Plant part to sample | Stage of growth | Number of plants |
|---|---|---|---|
| Corn or Grain sorghum | All above-ground | Seedling stage (less then 12’) | 20-30 |
| Corn or Grain sorghum | Top fully developed leaf | Prior to tasseling | 15-25 |
| Corn | Leaf at ear node | Tasseling to early silk* | 15-25 |
| Grain Sorghum | Second leaf from top | At heading | 15-25 |
| Soybeans | All aboveground | Seedling stage (less than 12") | 20-30 |
| Soybeans | Top fully developed trifoliate leaves | Prior to or during initial flowering* | 20-30 |
| Small Grain | All aboveground | Seedling stage (prior to tillering) | 50-100 |
| Small Grain | All aboveground | As head emerges from boot* | 15-25 |
| Peanuts | All aboveground | Seedling stage | 20-30 |
| Peanuts | Upper stems and leaves | Early pegging | 15-25 |
| Alfalfa | All aboveground | Prior to bloom | 30-40 |
| Alfalfa | Top third of plant | At bloom* | 15-25 |
| Bermudagrass | Whole plant top | 4 to 5 weeks after clipping* | 15-25 |
| Cotton | Whole plants | Early growth | 20-30 |
| Cotton | Petioles of youngest fully expanded leaves | During bloom* | 20-30 |
*Recommended sampling period for fertilizer evaluation.
Preparing for No-till Production Systems
While the decision to switch from conventional tillage systems to no-till production can be challenging in many aspects, several soil components need to be addressed prior to this switch. One of the biggest issues to be addressed is deep profile soil pH. Soil pH issues at depth can drastically limit overall root development into deeper soils, limiting access to potential nutrients and moisture lower in the soil profile. While lime can be applied to a no-till system, the ability for neutralization is limited without incorporation. Therefore, these deeper soil pH issues should be remedied prior to moving into no-till production.
Soil Sampling in No-till Production Systems
Soil sampling between no-till production systems and conventionally tilled production systems can vary drastically or be quite similar, all depending on management of the system and issues to be addressed. One of the most noted soil fertility characteristic of no-till production is nutrient stratification (or the formation of layers of that are non-uniform with depth in the soil). While this historically has been seen as a negative characteristic of no-till production, research has suggested very little impact for most nutrients managed within typical production systems.
The major issue with nutrient stratification in no-till production is soil pH. If soil pH was rectified prior to implementation of no-till production, little short-term issues should arise at depth with pH. The major issue comes at the surface. This especially is true in systems that have had continual surface applications of urea- and/or ammonium-based fertilizers. This fertilizer will undergo a transformation process in the soil that can decrease the overall soil pH.
Since these are issues at the surface of the soil, these should be able to be corrected rather easily. However, the identification of these issues from a traditional 0-to-6-inch soil sample can result in no application when one would be justified. Therefore, a sample of 0 to 6 inches and a 0 to 2 inches sample would be encouraged. These varied depths allow for identification of issues within the traditional soil zone (0 to 6 inches) as well as potential issues due to nutrient stratification (0 to 2 inches). One issue with collection of samples from only a 2-inch section of soil is collecting enough soil to get an adequate sample. Twenty cores typically are suggested for a traditional sample to get a representative sample across the field. However, it always is better to collect too much than not enough. The second thing to be considered is the current recommendations for lime application for correction of soil pH is based on a 0- to 6-inch sample. Since neutralization of soil acidity will not occur at these lower depths, recommendations can overestimate the amount of lime needed. Therefore, it often is recommended to lower the lime application rate by half to a third in no-till for a 0- to 2-inch sample.
The other major difference between soil sampling in no-till systems are production systems that have had banded fertilizer. The primary issue from no-till banded fertilizers is not the no-till nor the banding, but the combination of the two. When producers band fertilizers in a conventionally tilled system, the band does not behave any differently in season. However, without tillage to mix the banded and non-banded fertilizer together, a soil sample collected within these bands can grossly overestimate the concentrations of nutrients in the soil system. This can lead to an under-application of nutrient, which could result in a critical yield loss. Additionally, this issue typically is associated with phosphorus within the soil system but other nutrients, especially non-mobile nutrients, can be a concern as well. For sampling in fields that have had banded fertilizer in the past, the collector needs to ask a couple of questions to achieve a proper soil sample.
First, what crop with what row spacing has been previously planted? If the row spacing
is narrower than 12 inches, a normal sampling pattern can be used to collect a proper
sample. If the row-spacing is wider, is it known where the previous bands have been
placed? Will the successive crops be planted over the previous rows? When planting
over the previous row, collection of samples should be focused around these rows.
This sampling will provide an indication of the residual fertilizer left from the
banding and what nutrients were not taken up by the previous crop. If the previous
rows are known (along with the bands) but it is not known what successive crop will
not be planted over the previous rows, sampling must be done to estimate residual
fertilizer in band and outside of the previous bands. This will involve the collection
of soil from both these locations. However, as high potential residual levels of fertilizers
still within the band to drastically skew the results, a proper ratio of soil from
inside and outside the bands must be collected. For example, if the previous crop
was on 30-inch row-spacing, for every one sample collected within the banded zone,
20 samples need to be collected outside. The final scenario is if the previous rows
are not known. This can be the most challenging as the collection of soil is essentially
blind to where the previous banded rows. The best method to collection is to collect
a sample and conducted a paired collect half the distance of the previous row-spacing.
For example, if 30-inch row spacing was previously used collect a sample from a location
and collect a paired sample 15 inches from the previous sample in the direction thought
to be across rows. These paired samples would still be considered a single sample.
Therefore, a 20-core sample would consist of 40 individual cores or 20 paired cores.
Boron Deficiency
Calcium Deficiency
Chloride Deficiency
Copper Deficiency

Iron Deficiency
Magnesium Deficiency
Manganese Deficiency
Molybdenum Deficiency
Nitrogen Deficiency
Phosphorus Deficiency
Potassium Deficiency
Sulfur Deficiency
Zinc Deficiency
Chapter 5 - Fertilizer Use in Oklahoma
Brian Arnall
Fertilizer Use
It was not until 1945 that fertilization became a common practice for grain production in Oklahoma. This is illustrated in Figure 5.1 along with the average wheat yields from 1890 to 2004. Fertilizer use did not increase dramatically until the early 1960’s. From 1960 to 1980, the total tonnage of fertilizer sold in Oklahoma increased from 100,000 to 700,000 tons. Presently, almost 1,000,000 tons of fertilizers are sold annually in Oklahoma (Figure 5.1). It is important to note that this represents the total amount of fertilizer sold in Oklahoma and does not represent the amount used per acre.
Figure 5.1. Total fertilizer sold (tons) and average wheat yields in Oklahoma from 1890-2004.
The total volume of phosphorus and potassium fertilizers sold has not increased to any great extent since 1970 in fact it has been in, however, nitrogen fertilizer use continued to increase until the 1990’s (Figure 5.2). This demonstrates the importance of nitrogen fertilizers in the state and the relative use of nitrogen compared to phosphorus and potassium. When looking at sales since 1980 all three nutrients have been in decline (Figure 5.3) this decline is at a rate 1,700, 2,250, and 1,200 tons of N, P, and K fertilizer per year. This decline in fertilizer sales however is closely related to the total acres of wheat planted in the state (Figure 5.4).
Figure 5.2. Fertilizer nitrogen, phosphorus and potassium sold in Oklahoma, 1951-2014.
Figure 5.3. Fertilizer nitrogen, phosphorus and potassium sold in Oklahoma, 1980-2014.
Figure 5.4. Acres of wheat planted and phosphorus and potassium fertilizer sold in Oklahoma, 1980-2014.
From 1977 to the mid 1990’s, anhydrous ammonia (82-0-0) was the major source of N used in the state of Oklahoma. Since that time period there has been a marked increase in the use of urea ammonium-nitrate and urea sources of N, with urea being the top seller in recent years very closely followed by UAN (Figure 5.5). The use of ammonium-nitrate has decreased over this same time period while the contribution of N from diammonium phosphate has remained constant. Similar to anhydrous ammonia as an N source, diammonium phosphate (DAP) has remained the principle source of P (Figure 5.6). All other P sources combined contribute less than one third of the total P used in Oklahoma (Figure 5.6). However, there has been a tendency for ammonium polyphosphate (APP) to increase since mid 2000’s.
Figure 5.5. Amount of nitrogen fertilizer, separated by source, sold in Oklahoma, 1977-2014. Anhydrous ammonia (AA) 82-0-0, Ammonium Nitrate (AN) 34-0-0, Di-ammonium Phosphate (DAP) 18-46-0, Urea 46-0-0, and Urea Ammonium Nitrate (UAN) 28/32-0-0.
Figure 5.6. Amount of phosphorus fertilizer, separated by source sold in Oklahoma, 197-2014. Mono-ammonium Phosphate (MAP) 11-52-0, Di-ammonium Phosphate (DAP) 18-46-0, Ammonium Poly-phosphate (APP) 10-34-0.
Native Fertility
The lack of commercial fertilizer use before 1950 was largely due to the native fertility of the Oklahoma prairie soils which were not cultivated until the late 1800’s. Many of these soils were very fertile and required no added fertilizers in the first years of wheat production.
However, with time nutrients were continually depleted from the organic matter pool thus requiring fertilizers additions in later years. The demand for fertilizers was essentially a function of need. Continuous cultivation of these soils lowered soil organic matter levels from 4% (grass first turned over) to their present level of about 1%. Under continuous wheat production, this represented an annual depletion of the soil organic matter by 0.04%. However, this lowering of the soil organic matter was much greater in magnitude in early years and much less in later years. It is important to note that soils with 1% organic matter have about 2000 pounds of actual N in the top foot of soil. Therefore, almost 8000 pounds of N were present in these soils when they were first plowed. At that level one would think that there would never be a need for N, however, it must be remembered that this was N in an organic fraction. The amount of N that would be mineralized (biologically and chemically transformed to an available form for the plant) in the first 10 years was much greater than it is today. In addition, the crop needs for N were much less in the early 1900’s since varieties had much lower yield potentials and thus removed less N from the soil (Figure 5.1). Soils with 1% organic matter will mineralize less than 20 pounds of N per year and as such will not make a major contribution to the N needs for wheat grain production. However, in earlier years, demands for fertilizer N were less since the organic matter decay provided for most of the crop N needs.
Although this discussion has focused on nitrogen, it should also be noted that with time, the organic matter nutrient pool was also depleted of the other essential elements required for plant growth. With time, micronutrient deficiencies are expected to appear in isolated regions where continuous cropping has taken place for long periods of time.
Importance of Fertilizer Use
It is important to realize that many farmers in the developing world still do not apply fertilizers. In many of these impoverished areas, farmers burn down the forested areas, plant and produce crops for 10 to 20 years and then move on to another area of land. These are migrant farmers that have an average farm size of 2 acres, and who are extremely poor. The importance of this type of ‘slash and burn’ agriculture is that it only lasts until the nutrient supplying power of the ash from burned trees and brush, and the organic matter pool is depleted to the point where crops can no longer be produced. Not having availability to fertilizers, or more importantly the funds to apply any inputs to their farming techniques, they moved on to another forested area where they would cut down the trees, burn them, and produce crops for another 20 years or so until production was again stifled by depleted nutrient levels. Our agricultural systems are obviously much different from that of third world countries, however, organic matter depletion in this country is the same as that found elsewhere. Our farmers cannot move from one area to the next simply because the lands became increasingly unproductive with time, but rather must search for the methods and techniques to sustain production on the same lands.
Conventional Materials and Sources
Before World War II nearly all commercial fertilizer materials sold in the U.S. were dry materials. Dry fertilizer materials are either straight materials (those containing only one nutrient) or mixtures (those containing two or more nutrients). Mixed dry materials are available in two forms: 1) chemical compounds in which 2 of the major fertilizer elements are combined together in the granule and 2) bulk blends in which straight materials and/or chemical compounds are physically blended to make various grades. Bulk blending increased rapidly in Oklahoma during the early 1960’s and was readily accepted by growers because the proper ratio of fertilizer elements can be blended to fit soil test requirements. In Oklahoma, most dry blends are made from combinations of the following: ammonium nitrate, urea, diammonium or monoammonium phosphate and/or concentrated superphosphate, and muriate of potash. A blender with 4 to 5 bins of bulk, straight materials can blend most any ratio of material needed. A computer program is available to assist in the calculation of the needed ingredients for a particular blend at: http://www.soiltesting.okstate.edu/Interpretation.htm.
The major dry and liquid fertilizer materials available in Oklahoma are listed in Table 5.1.
Nitrogen Fertilizers
Anhydrous Ammonia, NH3, 82% N. Nitrogen was one of the first nutrients to be produced in a liquid form (liquid under pressure). Nitrogen is taken from the air and reacted with a hydrogen source in the presence of a catalyst to produce anhydrous ammonia. Virtually all nitrogen manufacturing facilities use natural gas as a source of hydrogen. Approximately 33,000 cubic feet of natural gas are required to produce a ton of ammonia.
Under pressure, anhydrous ammonia becomes a liquid that returns to a gas when released from the storage container. To prevent excessive loss of N, it must be injected into the soil and sealed until ammonium (NH4+) is formed. Anhydrous ammonia is a hazardous material and care must be taken in handling to avoid exposing human, animal or plant life to direct contact with liquid or gaseous forms. In nitrogen producing plants, anhydrous ammonia is the basic material used to produce other kinds of nitrogen fertilizers.
Urea ammonium-nitrate, 28-32% N. A common liquid N fertilizer is made from soluble urea and ammonium nitrate mixed in equal parts with water to form non-pressure N solution containing 28 to 32 percent nitrogen. Ammonium nitrate or urea solution, alone, can only be handled satisfactorily in the field, in approximately 20% N concentrations.
Nitrogen solutions that do not contain free ammonia can be applied to the soil surface without loss of N, although incorporation is recommended where ammonia volatilization loss from urea may be a problem. Ammonia free N solutions can also be applied in sprinkler irrigation systems with good success. Non-pressure N solutions are probably the most versatile of all N materials for application to a broad range of crops with a wide variety of application equipment.
Like any salt solution, nitrogen solutions will salt out. Salting out is simply the precipitation of the dissolved salts when the temperature drops to a certain degree. The salting out is determined by the amount and kind of salts in solution. As a general guide, 28% non-pressure solution salts out at about 0°F and 32% salt out at about 32°F, although this can vary between the materials produced by different manufacturers.
Corrosion inhibitors and a pH near 7.0 in nitrogen solutions reduce corrosion of carbon (mild) steel. The following materials are satisfactory for storing and handling nitrogen solutions: aluminum, stainless steel, rubber, neoprene, polyethylene, vinyl resins, glass and carbon steel. Materials that will be destroyed rapidly include copper, brass, bronze, zinc, galvanized metal, and concrete.
Ammonium Nitrate, NH4NO3, 33.5-34% N. Ammonium nitrate is made by reacting anhydrous ammonia and nitric acid. Half of the total nitrogen in the material is in the nitrate form and half is in the ammoniacal form. Most ammonium nitrate is prilled and coated.
Urea, (NH2)2CO, 45-46% N. Urea is formed by reacting ammonia and carbon dioxide. All of the nitrogen in urea is in the ammoniacal form. Urea is produced in both prilled and granular forms. It is classed as an organic compound since it contains carbon.
Ammonium Sulfate, (NH4)2SO4, 20.5-21% N. Ammonium sulfate is formed by reacting ammonia with sulfuric acid. All of the material’s nitrogen is in the ammoniacal form. Ammonium sulfate is an effective source of sulfur since it contains 24 percent S. It is produced in both crystalline and granular forms.
Table 5.1. Major fertilizer sources of nitrogen, phosphorus and potassium sold in Oklahoma.
| Source | Nutrient Composition | ||||||
|---|---|---|---|---|---|---|---|
| N (%) | P2O5 (%) | K2O (%) | CaO (%) | MgO (%) | S (%) | Cl (%) | |
| Nitrogen | |||||||
| Ammonium sulfate | 21 | - | - | - | - | 24 | - |
| Anhydrous ammonia | 82 | - | - | - | - | - | - |
| Ammonium nitrate | 33-34 | - | - | - | - | - | - |
| Calcium nitrate | 15 | - | - | 34 | - | - | - |
| Urea | 45-46 | - | - | - | - | - | - |
| Urea-ammonium nitrate (solution) | 28-32 | - | - | - | - | - | - |
| Phosphorus | |||||||
| Monoammonium phosphate (MAP) | 11 | 48-55 | - | 2 | 0.5 | 1-3 | - |
| Diammonium phosphate (DAP) | 18-21 | 46-54 | - | - | - | - | - |
| Ammonium polyphosphate (solution) (APP) | 10-11 | 34-37 | - | - | - | - | - |
| Urea-phosphate | 17 | 43-44 | - | - | - | - | - |
| Ordinary superphosphate* | - | 26-23 | - | 18-21 | - | 11-12 | - |
| Conc. (triple) superphosphate (TSP) | - | 44-53 | - | 12-14 | - | 0-1 | - |
| Rock phosphate* | - | 25-40 | - | 33-36 | - | - | - |
| Potassium | |||||||
| Potassium chloride | - | - | 60-62 | - | - | - | 47 |
| Potassium sulfate | - | - | 50-52 | - | - | 17 | - |
*no longer important sources in Oklahoma
Phosphorus Fertilizers
Diammonium Phosphate, DAP, (NH4)2HPO4, 18% N, 46% P2O5. This popular N-P material is produced by reacting ammonia and phosphoric acid. All of the nitrogen is in the ammoniacal form and the P is highly water-soluble. It is produced in the granular form.
Monoammonium Phosphate, MAP, NH4H2PO4, 11-12% N, 48-60% P2O5. This material is produced by reacting ammonia and phosphoric acid. All of the N is in the ammoniacal form and the P is highly water-soluble. Most MAP is produced in the granular form.
Phosphoric Acid and Superphosphoric Acid, 54-85% P2O5. Phosphate rock deposits are the basic source of all phosphate materials. The principal world reserves are located in North Africa, North America and the former Soviet Union. The primary intermediate step in the production of phosphorus fertilizers is phosphoric acid. In some areas, phosphoric acid is applied to the soil as a form of fertilizer; however, the handling problems associated with this acid has limited its use.
In fluid fertilizer production two types of acid are commonly used; ortho phosphoric (phosphoric acid) containing about 54% phosphorus (P2O5) and superphosphoric (polyphosphoric acid) containing up to 85% phosphorus (P2O5). Being more concentrated, it is possible to produce a higher analysis P fertilizer from superphosphoric acid.
When ortho phosphoric acid is reacted with ammonia, the acid can be neutralized to a pH of about 6.5 to produce a nitrogen phosphorous solution of 8-24-0. This was the basic phosphorous material used in mixed liquid fertilizers for several years. The development of superphosphoric production procedures make it possible to produce the higher analysis nitrogen phosphorous solutions (10-34-0), currently used as the basic phosphorous source in liquid and suspension grades of liquid fertilizer.
Ammonium Polyphosphate Solutions, APP, 10% N, 34% P2O5. The ability to produce 10-34-0 ammonium polyphosphate solution played an important role in the rapid growth of liquid N-P-K fertilizers during the 1960’s. Improved storage and application equipment and other technical advances have enabled this growth to continue.
Ammonium polyphosphate solutions can contain up to 70 percent of the total P2O5 as a poly-P form. The remaining P2O5 is as an orthophosphate. All phosphate fertilizers contain some orthophosphate with many being 100% in the ortho form. In fluids, it is generally accepted that high poly content, above 55 percent, improves storage quality and the opportunity to carry low cost sources of micronutrient metals in liquid grades.
Ordinary Superphosphate, 20% P2O5. Ordinary superphosphate is made by treating finely ground phosphate rock with sulfuric acid. The P2O5 content of this source ranges between 18 and 22 percent. This source has between 11 and 12 percent sulfur as calcium sulfate and is sold as granular form. This low analysis material is no longer readily available in Oklahoma.
Concentrated Superphosphate, 46% P2O5. This source is produced by treating ground rock phosphate with phosphoric acid. The product will vary from 42-46 percent P2O5 with the most common analysis 46% P2O5.
Potassium Fertilizers
Potassium (K) is found throughout the world in both soluble and insoluble forms. The soluble forms are the principal form used in fertilizers. Potassium chloride is by far the most important source of fertilizer K.
Potassium Chloride (Muriate of Potash), KCl, 60% K2O. This is the K salt of hydrochloric (muriatic) acid. Most potash deposits are in this form. It is the most popular potash material used in fertilizers. Muriate of potash is a crystalline material. It is available in various particle sizes which are chosen to coincide with other materials for bulk blending. Some muriate of potash contains iron coatings, giving it a reddish color. Most muriate of potash is white or translucent. Color or particle size does not affect potassium availability for plant growth since it is a water soluble compound. In addition, potassium chloride is the major source of potash for liquid fertilizers. The fine soluble 0-0-62 grade is used for both liquid and suspension. About 10% K2O is the maximum that can be dissolved in a liquid but up to 30% K2O can be carried in a suspension.
Potassium Sulfate, K2SO4, 50% K2O. Like muriate of potash, potassium sulfate occurs naturally in limited deposits. It is extensively used in tobacco fertilizers where there is concern regarding chlorine build-up. It contains 17 percent sulfur and is widely used in areas where both potassium and sulfur are needed. Potassium sulfate has a lower solubility than KCl and is primarily used in suspensions to produce chloride free potassium and sulfur.
Secondary Elements
Calcium (Ca). Calcium fertilizers are not usually needed in Oklahoma. Common sources of supplemental Ca are lime and gypsum.
Calcium Carbonate (Lime) 20-40% Ca
Calcium Sulfate (Gypsum) 23% Ca, (18.6% Sulfur)
Normal Superphosphate 22% Ca, (20% P2O5, 12% Sulfur)
Magnesium (Mg). The most common sources of magnesium are magnesium sulfate and dolomitic lime.
Magnesium Oxide 52% Mg
Magnesium Sulfate 16% Mg
Potassium - Magnesium Sulfate 11% Mg, (22% K2O, 22% Sulfur)
(Sul-Po-Mag, K-Mag)
Dolomitic Limestone (varies) 12% Mg
Sulfur (S). Sulfur is most available when supplied in the highly water soluble sulfate form. Ag. sulfur (elemental sulfur) can be used, but requires biological oxidation over time to convert the elemental form to available sulfate.
Calcium Sulfate (Gypsum) 17% S (22% Ca)
Potassium Sulfate 17% S
Sulfate of Potash, Magnesia 22% S
Ammonium Sulfate 24% S
Normal Superphosphate 12% S
Ammonium Thiosulfate 26% S
Boron (B). A sodium borate (solubor) containing about 20% B is the source of B most commonly used in liquids. Boric acid and other soluble forms containing between 14 to 20% B are also suitable for liquid mixes.
Borax 11.3% B
Zinc (Zn), Iron (Fe), Copper (Cu), and Manganese (Mn)
The micronutrient elements can be discussed as a group since their sources are somewhat similar. Industry separates the compounds into two general categories; inorganic and organic. Inorganic include sulfates, oxides, carbonates and chlorides. The term organic applies primarily to chelated products and some sequestered materials. Most chelates, and particularly liquid products, can be mixed with liquid without difficulty.
Zinc
Zinc Sulfate 25-36% Zn
Zinc Oxide 50-80% Zn
Zinc Chloride 48% Zn
Zinc Chelate 9-14.5% Zn
Iron
Ferrous Sulfate 20.1% Fe
Ferric Sulfate 19.9% Fe
Ferrous Ammonium Sulfate 14.2% Fe
Ferric Chloride 34.4% Fe
Iron Chelate 10% Fe
Copper
Copper Sulfate 25% Cu
Manganese
Manganese Sulfate 23-28% Mn
Molybdenum (Mo). Ammonium molybdate is satisfactory for liquids. Sodium molybdate can also be used although it is less soluble than ammonium molybdate. Since Mo is applied in ounces per acre, liquids are ideal for getting even distribution.
Sodium Molybdate 39.7% Mo
Ammonium Molybdate 54.3% Mo
Chlorine. Chlorine has only recently been found deficient in Oklahoma soils. The deficiency in wheat on deep sandy soils near Perkins, OK can be corrected using muriate of potash (0-0-60). This is the common source of potassium, which is usually also deficient in these sandy soils.
Mixed Fertilizers
Fertilizer mixtures account for a significant portion of the total amount of fertilizer consumed in Oklahoma. These mixtures are either manufactured at large granulation plants and shipped to the dealer as the grade or they are blended by local blend plants. Field research has shown little or no differences between the chemical granulated materials and physical blends unless segregation occurs in the blends.
Methods of Application
Comprehensive evaluation of fertilizer placement research reveals that no single question has been asked so many times for so many different crops and production systems as the question of whether to "band or broadcast". Interestingly, it remains an important question today and may well be in the future. The most common method of applying fertilizers in modern times has been to broadcast, either with or without incorporation. However, the method used depends on various factors including the fertilizer to be applied, tillage, equipment available and crop grown.
Banding
Banding immobile nutrients such as P has become a common method for soils with high fixation capacities. In general, banding is the placement of fertilizer nutrients in a concentrated zone near the seed. Initial reasons for banding were:
- to reduce the surface area of the fertilizer in direct contact with the soil, and thus minimize fertilizer-soil reactions that reduce chemical availability;
- to apply the nutrient where there is the greatest chance for root contact.
Banding will likely have little beneficial effect for mobile nutrients such as N and S. Banding P and K has been beneficial where starter effects were desired in cool, wet climates. Recent work has shown banding P with the seed at planting on highly acid soils can reduce aluminum toxicity.
Soluble fertilizers placed in a band may cause germination and/or seedling injury if rates are too high. In general, the salt index (applied N + K2O + ½ S) should not exceed 30 lb/ac for wheat and 7 lb/ac for corn. These two rates are based on 6” row spacing and wheat and 30” row spacing in corn. The row spacing at which any crop is planted impacts the safe salt index rate (Table 5.2) In extremely arid regions and/or where rapid drying takes place, salt rates less than these can adversely affect crop seed germination. Although banding P with the seed has become popular for Oklahoma wheat farmers with acid soil, it remains as a temporary alternative to liming.
| 6" | 7.5" | 10" | 12" | 15" | 20" | 30" | |
|---|---|---|---|---|---|---|---|
| Wheat | 30 | 24 | 18 | 15 | |||
| Canola | 10 | 8 | 6 | 5 | 4 | 3 | 2 |
| Sorghum | 25 | 20 | 15 | 12.5 | 10 | 7.5 | 5 |
| Corn | 14 | 10.5 | 7 |
Unlike broadcasting, there are several variations of band applications including with the seed, below the seed, beside the seed, dribble surface bands, spoke tooth bands, spot placement, point injection, and dual band applications. Accurate characterization of band applications must also consider spacing, form (liquid or solid), and depth of placement. An illustration of plant response to banding is found in Figure 5.7. Roots respond to increased P availability, increasing in growth within the band where the P is placed. If a soil were deficient in P, all roots would not explore the entire soil profile in search of this limiting element. Instead, some roots penetrate the band or localized area where P has been applied, and proliferate in that zone (Figure 5.7).
Broadcast
Broadcast applications of granular fertilizers are most often applied prior to planting. For many grain producers, this method of application can be more economical and requires less time, which can be important when one operator must cover a large acreage. However, poor distribution patterns from bulk dry spreaders can result in uneven stands and lower grain yields. Ultimately, it is up to the farmer to check commercial fertilizer applicators. Using sample pans (8 to 10 pans, 2 ft wide) spread across the application width, one can quickly assess the distribution pattern of the fertilizer applicator. If the weighed amounts in the pans differ by more than 10-15%, the application equipment should be adjusted accordingly. Applicators which can cover a broad width (30-60 feet with each pass), need close monitoring to avoid uneven distribution of the applied fertilizer.
Figure 5.7. Plant root development when P is banded in phosphorus deficient soils (conventional tillage).
Broadcast applications of phosphorus have proven to be satisfactory in minimum tillage crop production since this method of placement effectively reduces the surface area of the soil in contact with the fertilizer (Figure 5.8). The advantages of this method in reduced tillage crop production, at least under humid region cropping conditions is also a function of placing the fertilizer near the zone (surface horizon 0-2 in) where increased moisture and root mass are present. In this regard, broadcast applications of P in minimum tillage systems have been viewed as surface horizontal bands (Figure 5.8). Alternatively, localized band applications of P in conventional tillage have commonly increased uptake efficiencies and grain yields when compared to broadcast methods as a result of effectively reducing soil-fertilizer P fixation.
Figure 5.8. Plant root development when P is broadcast applied in minimum/zero tillage production systems.
Volatilization Losses from Surface Applied Urea and UAN Solutions
Urea is now the most widely used solid form of N in the world. Methods of applying urea forms of N in minimum tillage systems have been given considerable attention since gaseous losses of N as ammonia gas (NH3) are known to occur when urea is applied to soils with pH > 7.0 and where surface soil temperatures are high. Because of this problem, various researchers have stressed the importance of banding urea below the surface of the soil.
When urea is broadcast applied to soils where minimum or zero tillage is used, N losses as ammonia gas can increase due to accumulated surface residues. This is due in part to the enzyme urease (found in crop residues) which is responsible for the chemical transformation of urea ((NH2)2CO) to ammonium (NH4+) that can be used by the plant. Ammonium can be chemically transformed to ammonia gas (NH3) and lost from the soil. This loss is favored by application of urea to wet soil or residue surfaces that remain moist for several hours, followed by good drying conditions (windy, high temperature). Any loss decreases the amount of N available to the crop and increases the fertilizer requirement. Some of the surface applied N will stimulate microbial decay of residue and be "tied-up" in microbial tissue. Because of this, when urea is surface applied in reduced tillage systems, a higher rate of N is generally needed for optimum wheat grain yields when compared to conventional tillage. Sprayed applications of solutions 28 or 32 (UAN) on bermudagrass may also be less effective than other sources of nitrogen because of the high chance for ammonia from the urea to volatilize.
Reduced tillage systems have shown distinct advantages over that of conventional tillage in terms of soil erosion control, increased soil moisture, and higher residual soil mineral N levels. However reduced tillage systems can also increase volatilization losses from surface applied urea when compared to conventional tillage. Other disadvantages associated with reduced tillage systems include increased surface soil acidity, denitrification, immobilization, NO3-N leaching and higher N requirements for crop production.
In general, urea sources of N should not be broadcast when soil pH exceeds 7.0, and where minimum tillage/reduced tillage practices are employed.
Management Strategies to Increase N Use Efficiency
Fertilizer N use efficiency in crop production has been primarily influenced by volatilization losses, surface immobilization and NO3-N leaching beyond the rooting zone. Volatilization losses from applied urea have been effectively reduced by surface incorporation of urea-N sources. Other work has focused on the use of urease inhibitors that selectively inhibit the urease enzyme involved in ammonium hydrolysis. Surface immobilization of applied N can be reduced by using various forms of banding (localized placement).
Sidedress or Split Applications
The most practical method of reducing NO3-N leaching losses is to apply the N when it is needed most by the crop. Split applications can effectively reduce mobile nutrient leaching losses by applying the required amounts during high crop uptake stages. Fertilization practices mirror the initial ideas behind split applications by applying the same actual N rate in smaller quantities over time and in relation to crop need. Nitrate-N leaching has also been reduced in certain areas by the use of nitrification inhibitors which slow down the transformation of NH4+ to NO3-. This is accomplished by the selective inhibition of the bacteria nitrosomonas sp. involved in the biological oxidation of NH4+.
Knife Injection of Anhydrous Ammonia
Depending on the soil, anhydrous ammonia should generally be applied 4 to 8 inches below the soil surface. Slower tractor speeds can favor better ammonia retention by the soil (and less loss of ammonia gas) due to improved soil closure behind the knife applicator. If soils are too dry and large chunks of soil form behind the applicator, or too wet and a trench forms, then the resulting poor seal allows much of the ammonia gas to escape to the air. Spacing of the applicator knifes should be based on the row spacing to be used, rate of application and whether the application is made before planting. The minimum practical spacing is 14 inches and the maximum is 40 inches.
When anhydrous ammonia is applied sidedress within row crops, the knives should be placed to travel 6 to 10 inches to the side of the row. For other crops with extensive root systems, the knives should be spaced to travel between the rows. On soils with extremely high clay contents, and/or very sandy soils, anhydrous ammonia may not be a suitable N source due to gaseous losses which can occur. In general, ammonia losses are minimized when soil moisture content is between 12 and 18% (Figure 5.9). It is also important to note that at the 9 and 12 inch depths of placement, ammonia losses are further reduced. However, it is not advisable to knife anhydrous ammonia at depths greater than 9 inches due to equipment wear and increased fuel costs.
The long-term benefits of knifing anhydrous ammonia preplant compared to other more costly granular and liquid N forms has been noted in wheat, corn and sorghum production. Similar results from using anhydrous ammonia on other crops is largely due to the lower cost per pound of N and economies of scale when considering the cost of anhydrous ammonia versus alternative N sources. Additionally, application costs may be nil when done in conjunction with a planned tillage operation.
Figure 5.9. Relationship of ammonia loss and soil moisture at the time of application using different depths of placement.
Chapter 6 - Use of Animal Manure as Fertilizer
Hailin Zhang
Introduction
Animal production is a large segment of the economy of Oklahoma. Confined animal feeding operations produce large quantities of manure requiring proper management. Animal waste has been used by ancient and modern farmers to enhance crop production and improve soil health. Besides providing valuable macro- and micro-nutrients to the soil, manure supplies organic matter to improve soil tilth, improves infiltration of water and retention of nutrients, reduces wind and water erosion, and promotes growth of beneficial organisms. Therefore, manure land application recycles nutrients and sustains crop production (Figure 6.1).
Manure applications, however, may cause surface and groundwater pollution if mismanaged. Surface runoff from manured land may contain plant nutrients and organic materials. Excess nutrients and organic material in surface water often causes algal bloom, which increase the turbidity and biological oxygen demand of water. The polluted water may cause odors and result in a fish kill if the dissolved oxygen is significantly lowered. Excessive applications of manure may also cause nitrate-nitrogen (NO3-N) to accumulate in the soil. The excess NO3-N can reach the surface water through drainage ditches or groundwater through leaching.
Figure 6.1. Land application of animal manure recycles nutrients back to the land. It is the most economical and environmentally sound method to handle by-products in meat and milk production.
This chapter is to provide agronomic information for the efficient use of manure nutrients for crop production and to help protect surface and groundwater quality. A work sheet is also provided for calculating the agronomic rate of manure application depending on your crop yield goal and soil conditions.
Manure Management Functions
An agricultural waste management system designed for a confined animal feeding operation consists of six basic functions of manure management: production, collection, storage, treatment, transfer, and utilization (Figure 6.2). It is important to understand each of these functions since they affect the nutrient contents of the manure.
Production
Production is the function of the amount and nature of manure generated by a livestock or poultry operation. Oklahoma farms produce about 9 million tons of manure from CAFOs each year. The generation of unnecessary waste should be kept to a minimum. Leaking watering facilities and spilled feed contribute to the production of waste. These problems can be reduced by careful management and maintenance of feeders, watering facilities and associated equipment.
Collection
This refers to the initial capture and gathering of the waste from the point of origin or deposition to a collection point.
Storage
Storage is the temporary containment of the waste. The storage facility of a waste management system is the tool that gives farmers control over scheduling of transfer operation or land application.
Treatment
Treatment is any process designed to reduce pollution potential of the waste, including physical, biological, and chemical treatment. It includes activities that sometimes are called pretreatment, such as the separation of solids.
Transfer
Transfer refers to the movement and transportation of the waste throughout the system. It includes the transfer of the waste from the collection point to the storage facility, to the treatment facility, or to the utilization site. Waste may require transfer as a solid, liquid, or slurry, depending on the total solid concentration.
Utilization
Utilization refers to the recycle of waste products into the environment. Agricultural waste may be used as a source of energy, bedding, animal feed, mulch, organic matter or plant nutrients. Properly treated, they can be marketable. Most often they are land applied as soil amendments, therefore, utilization of manure as plant nutrients will be discussed here in detail.
Figure 6.2. Manure Management Functions. (NRCS)
Value of Animal Manure
Animal manure contains valuable nutrients that can support crop production and enhance soil chemical, physical and biological properties. Thus, manure can be an asset to a livestock production operation if its nutrient value is maximized. Nutrient composition of farm manure varies widely even for the same species of animal. In the past, manure was primarily solids, thus application was a problem because it required handling a large tonnage of low-analysis material. Today, an increasing amount of the waste is fluid, and analysis is lower because of the higher water content. The approximate fertilizer values for various manures are shown in Table 6.1. However, the actual value is based on the need for nutrients. For example, crops will not benefit from additional phosphorus if the field is already high in soil test phosphorus. These nutrients are average values and a chemical analysis on each sample should be obtained before manure is applied to your field. Manure sampling procedures and analysis available through OSU Soil, Water and Forage Analytical Laboratory (soiltesting.okstate.edu) will be discussed later in this guide.
| Manure Type | Dry Matter % | Total N (lbs/ton) | P2O5 (lbs/ton) | K2O (lbs/ton) | Value* $ |
|---|---|---|---|---|---|
| Feedlot Manure | 62 | 24 | 21 | 25 | 30.2 |
| Poultry Litter | 77 | 63 | 61 | 50 | 78.6 |
| Total N (lbs/1,000 gallons) | P2O5 (lbs/1,000 gallons) | K2O (lbs/1,000 gallons) | |||
| Lagoon Effluent | 0.5 | 4.2 | 1.0 | 5.0 | 4.24 |
| Lagoon Sludge | 7 | 15 | 16 | 11 | 18 |
| Dairy Slurry | 3 | 13 | 11 | 11 | 14.9 |
* Based on a per-pound value of $0.50 for available nitrogen (assuming 60 percent is available), $0.50 for P2O5, and $0.50 for K2O
Methods of Land Application
Manure can be applied to a land by surface broadcasting using a manure spreader, by spreading with an irrigation system, or by tank wagon followed by plowing or disking, by broadcasting without incorporation or by knifing under the soil surface. Research has shown maximum nutrient benefit is realized when manure is incorporated into the soil immediately after application.
Immediate incorporation of solid manure minimizes nitrogen loss to the air and allows soil microorganisms to start decomposing the organic fraction of the manure. This increases the amount of available nitrogen to the crop. With liquid manure systems, the practice of injecting, chiseling, or knifing the manure beneath the soil surface reduces nitrogen losses by volatilization and potential runoff. Incorporation of either solid or liquid manure also reduces odor problems. Large nitrogen losses usually result from application by irrigation equipment. Actual losses depend on NH4-N content, and increase as the irrigation water pH increases. Nitrogen loss by ammonia volatilization from surface applications is greater on dry, warm, windy days than on days that are humid and/or cold. That means loss generally is higher during the late spring and summer seasons than it is in the late fall and winter. It is especially important poultry and veal calf manure be incorporated into the soil as soon as possible after application because of its high pH (alkalinity). To prevent local high concentrations of ammonium or inorganic salts, which can reduce germination and affect yields, manure should be applied uniformly.
Phosphorus and potassium, unlike nitrogen, are not subject to either volatilization. Incorporation of manure, however, will minimize phosphorus and potassium losses due to runoff, and increase their agronomic value.
Procedures for Sampling and Analyzing Manure
The actual nutrient value of manure from a particular operation will differ considerably due to the method of collection and storage. For accurate rate calculations, it is strongly recommended that the nutrient content of manure be determined by laboratory analysis annually or when manure handling procedure changes. The analysis report should at least include information on dry matter, electrical conductivity, total nitrogen, phosphorus and potassium. Nitrate-N, ammonium-N and water soluble phosphorus needs to be determined sometimes.
How to Collect a Representative Sample?
The key to an accurate manure analysis is to obtain a representative sample by mixing the manure and using proper sampling techniques. A considerable amount of nitrogen can be lost if a sample is not correctly taken and handled.
For liquid manure storage facilities, samples may be collected by attaching a container, such as a jar or milk jug, to a long rod (such as a paint roller connected to a paint pole). If possible, agitate the contents of a manure pit to ensure a well-mixed sample. Liquid storage facilities have a tendency for the waste to stratify, with the solids settling to the bottom and the liquids remaining on top. Normally the nitrogen and potassium will be more concentrated in the top liquid, while the potassium will be concentrated in the bottom solids. Several sub-samples should be collected from the storage facility, placed in a bucket to make a composite sample, and mixed well by stirring. From this mixture, a quart-size plastic container is filled half full. Filling the bottle half full will allow for gas expansion of the sample and prevent the bottle from exploding. The sample should be kept frozen or as cold as possible until you can take it to your county extension office or ship it directly to a laboratory. Liquid samples also can be collected during land application. These samples best represent the amount of nutrients applied to the land. Randomly place catch pans in the field to collect the liquid as it is land being applied by an irrigation system or honey wagon. Immediately after the waste has been applied, collect the waste from catch pans and combine in a bucket to make one composite sample. Take the final sample from this mixture, and fill the container as described previously. Sampling waste this way accounts for nutrient losses due to both storage and handling, as well as losses due to application.
For solid manure, obtain samples from several parts of the manure source and place in a bucket to make a composite sample. Do not allow the material to dry, and take about 1 pound of final sample in a plastic bag, twist and tie tightly. For added safety, place in a second plastic bag. Preserve immediately by freezing.
Deliver the liquid or solid manure sample to the laboratory personally, or package thoroughly, in a strong, insulated container and ship the fastest way possible. Check with your county Extension educator for more details on how to collect samples and where to obtain an analysis.
Nutrient Availability of Manure to Crops
Not all nutrients present in manure are readily available to a crop in the year of application. To be used by plants, nutrients must be released from the organic matter in manure by microbial decomposition and into a chemical form that is soluble in water.
Most manure nitrogen is in ammonium (NH4+) and organic forms. Potentially, all of the ammonium-N (NH4-N) can be utilized by plants in the year of application. However, if manure is broadcast on the soil surface and not quickly incorporated, considerable NH4-N will be lost to the air as ammonia (NH3) gas increasing odor and lose valuable nitrogen, The ammonium added will be subject to nitrification resulting in rapid formation of nitrate-N (NO3-N). Nitrogen in the organic form must be converted (mineralized) into inorganic forms which are plant available (ammonium and nitrate) before it can be absorbed by roots. The amounts of organic nitrogen converted to plant-available forms during the first cropping year after application vary depending on both livestock species and manure handling systems. In general, about 30 to 70 percent of the organic nitrogen may become available the year of application. Organic nitrogen released during the 2nd, 3rd and 4th cropping years after application is usually about 50, 25 and 12.5 percent, respectively, of that mineralized in the initial season. Soil
test data should be used to determine the potential accumulation of nitrogen after repeated manure applications.
If soil organic matter levels are low, some nitrogen can be tied up (immobilized) in the soil and released in the subsequent years resulting in much less available the first year. In addition, manure contributes considerable organic matter to the soil and increases bacterial activity which can tie up inorganic nitrogen making it not immediately available to the growing plant. The average nitrogen available in the first year of application and in the consequent years is listed in Table 6.2.
| Manure Type | 1st Year Availability | Future Availability |
|---|---|---|
| Feedlot Manure | 50%-70% | 10%-20% |
| Poultry Litter | 50%-70% | 10%-15% |
| Dairy Manure | 50%-70% | 10%-20% |
| Swine Lagoon Effluent | 30%-50% | 5%-10% |
The availability of phosphorus and potassium in manure is considered similar to that in commercial fertilizer since the majority of phosphorus and potassium in manure is in the inorganic form. For all manure types, 90% of phosphorus and potassium in the manure are considered available during the first year of application and 10% for future years. Another management approach is to rotate the fields that receive manure if excess phosphorus is applied so it can be efficiently utilized in subsequent cropping seasons and phosphorus buildup in the soil is minimized.
Developing a Manure Application Plan
Some producers apply enough manure on the land to meet crop nutrient needs and then unnecessarily add commercial fertilizer. This practice not only wastes money and much of the manure’s potential value as a plant nutrient source, but also can cause nutrient imbalance in the soil and increase nutrient leaching or runoff into water sources. Repeated applications of excess manure result in a wasteful buildup of phosphorus and potassium in soils. Salt buildup also is possible if manure salt concentration is higher than normal, application rate is excessive, and rainfall is low.
Livestock and poultry producers should develop a manure nutrient management plan that first maximizes the use of manure nutrients and then supplements with commercial fertilizers only if additional nutrients are needed for the crop. The major elements of such a plan should include:
- periodic analysis of the manure produced in the animal operation
- a routine soil testing program
- keeping accurate records of fields manured and the application rates used
- sufficient storage capacity for timely application
- field availability for manure application
- uniform applications and proper timing of manure application across the entire field
- calibration of manure spreaders so application rates can be determined
- applying manure to meet crop nutrient needs based on realistic yield goals
- complying with state and federal regulations
Suggestions for Proper Land Applications
The following are some suggestions to help ensure safe and effective application of animal manure to cropland:
- When applying manure and waste water to a land, appropriate buffer areas should be used;
- Unless immediately incorporated into the soil, surface apply manure at reasonable distances from streams, ponds, open ditches, residences and public buildings to reduce runoff, odor problems and to avoid neighbor complaints;
- To minimize farmstead odor problems, spread raw manure frequently, especially during the summer. Spread early in the day when the air is warming and rising rather than blowing toward populated areas or when the air is still;
- When the soil is frozen or saturated, apply manure only to relatively level land where runoff will be less likely to occur;
- Agitate liquid manure thoroughly in pits to ensure removal of settled solids. This
is important for uniform application of the nutrients and for obtaining accurate,
representative analysis samples;
Consider irrigating with diluted manures (lagoon or runoff liquids) during dry weather to supply needed water as well as nutrient to growing crop; - Do not spread liquid manure on water-saturated soils where runoff is likely to occur;
- Make safety your first priority when removing manure from tanks or pits. Because of oxygen deficiency or toxic gas accumulation, remove animals or increase ventilation in slatted floor areas over manure pits during agitation.
Determining How Much Manure can be Applied
Land application rates should be based on the nutrient requirements of the crop being grown to ensure efficient use of manure nutrients and minimize the chances of leaching. Soil testing, manure analysis, irrigation water analysis, and proper estimation of yield goal are necessary to calculate proper agronomic application rates of manure and fertilizers. However, if manure analysis information is not available, the data in Table 6.1 and 6.2 or other sources may be used to calculate approximate application rates. Table 6.3 illustrates the steps to come up with an agronomic rate of manure application. This is what one should do to maximize the benefits of manure and minimize the impact on the environment. However, more manure may be allowed to apply. More information on manure rules and regulations is available from Oklahoma Department of Agriculture, Food and Forestry and the Oklahoma Natural Resource Conservation Services.
Oklahoma Cooperative Extension Services’ Manure and Animal Waste Management website also is a good source of information: animalwaste.okstate. edu.
Table 6.3. Manure Application Rate Calculation Worksheet.
Chapter 7 - Environmental Concerns Associated with Fertilizer Use
Chad Penn
Use of fertilizer has generated numerous environmental concerns in recent years. Concerns can be categorized by their effect on water quality, air quality, and human and animal health. In each case, the primary interest is nitrogen and phosphorus content, although others need to be considered, depending on the fertilizer source. As previously covered, there are many available fertilizer sources including commercial fertilizers, biosolids and animal waste. Environmental concerns become a potential hazard with the misuse of these materials. Misuse generally arises when fertilizer application rates exceed agronomic requirements. It is emphasized here application of fertilizer materials is not environmentally unsound, but excessive application of any of them can lead to potential hazards. In many states fertilizer use is now being regulated. In Oklahoma, phosphorus applications are regulated based on the NRCS “Phosphorus Index,” which limits phosphorus applications as a function of soil test phosphorus level, watershed and conditions. Therefore, producers should be aware of potential problems. By knowing the potential problems, producers can properly manage fertilizer inputs to maximize production yet minimize negative environmental impacts.
Nitrogen
Environmental concerns with nitrogen focus on water quality but also include air quality and human and animal health. Water-quality issues include nitrogen concentrations in surface water and groundwater. Concerns for surface water are related to nitrogen entering streams, ponds and lakes where elevated levels will stimulate algae growth resulting in algae blooms. Upon the death of the algae, microbial activity increases resulting in a decrease in available oxygen for biological functions, a condition referred to as eutrophication. Eutrophication has a detrimental effect on most aquatic species. It occurs when there are adequate sources of nutrients, but the system is limited by the available oxygen, resulting in the death of many aquatic species including fish and invertebrates.
The most common pathway for land-applied nitrogen to reach surface waters is by runoff waters. These waters often will contain soluble materials and soil sediments. Therefore, even nitrogen applied at agronomic rates and incorporated into the soil is susceptible to moving into surface waters by runoff when carried by soil particles. Nitrate-N is a soluble nitrogen form and ammonium-N can be attached to the soil particles as they are carried into the stream or impoundment. Several steps can be taken to minimize nitrogen problems associated with runoff from fields into surface waters. One of the most effective ways to minimize runnoff-related nitrogen problems is to maintain plant residue on the soil surface, which will enhance water infiltration and reduce the volume of water and amount of soil sediments moved from the field into surface water. Another effective practice is to leave a buffer strip of vegetation between the field and the surface water, which can act as a trap for many of the soil sediments. By catching sediments in the buffer strip the amount of nitrogen reaching the surface water is reduced.
Although eutrophication of surface waters is important, much of the regulation in other states focuses on the use of nitrogen in areas where a subsurface aquifer is within 10 feet of the soil surface. Nitrogen in the nitrate (NO3-) form is very susceptible to leaching through the soil profile as previously discussed, therefore, these sites possess a real possibility for elevated levels of NO3- to enter the aquifer when nitrogen application rates are in excess of agronomic rates. Concerns with nitrate reaching an aquifer generally are related to animal and human health rather than an imbalance in environmental nutrient requirements.
Methemoglobinemia (blue-baby syndrome) can result from the ingestion of nitrate in water or nitrate-rich food products. Ingested nitrate then can be reduced to nitrite in the upper gastrointestinal tract, and once incorporated in the blood system can form methemoglobin. Methemoglobin, unlike hemoglobin, cannot function as an oxygen carrier, ultimately resulting in anoxia or suffocation if high amounts are present. Infants younger than three months are highly susceptible to gastric bacterial nitrate reduction because they have very little gastric acid production and low activity of the enzyme that reduces methemoglobin back to hemoglobin.
Nitrogen-nitrosamines are potent carcinogens in animals. These compounds can be synthesized from amines and nitrous acid under certain conditions. When nitrate is reduced to nitrite, it can give rise to the formation of nitrogen-nitrosamine compounds that are an important class of chemical carcinogens for humans. However, nitrosamines occur in very few foods and at very low levels because of their chemical instability. It is important to note the presence of nitrosamines in food products generally is not associated with nitrates from nitrogen fertilizers, but rather the use of nitrite as a curing agent in meats, poultry and fish. Potassium nitrate also has been used as a food preservative. Other studies have shown an association between nitrate in drinking water and the incidence of gastric carcinoma in adults continuously exposed to high nitrate.
Agronomic solutions have been available for years to deal with fertilizer NO3-N pollution of surface and subsurface water supplies. Nitrogen fertilizer recommendations based on removal and use efficiency have been shown to be both environmentally sound and economical. Recent research by the OSU soil fertility project has demonstrated limited potential for NO3-N leaching when the recommended nitrogen fertilization rates are employed in continuous winter wheat. This work has also shown that nitrogen rates needed for maximum wheat grain yield can be exceeded by small amounts without increasing soil profile NO3-N accumulation.
The use of nitrogen in agriculture has been identified as a contributor to water pollution. However, it also has been found that this contribution to ground water contamination occurs when nitrogen is managed improperly. Under continuous production of wheat, applied nitrogen at the recommended rate (using soil testing and realistic yield goals) will not result in increased NO3-N contamination of groundwater. Also, the sensor-based system developed at OSU (discussed in Chapter 10) likely will decrease the risk of NO3-N contamination of groundwater, since this technology simulates soil testing, but on a much finer scale. By working at a sub-field scale, excessive nitrogen application can be reduced, thus reducing the risk of NO3-N leaching to groundwater.
A final concern related to the use of nitrogen fertilizers in some regions is air quality. This is primarily related to the application of animal manures and biosolids and resulting ammonia (NH3) that can be lost to the atmosphere from them. High concentrations of atmospheric NH3 is a potential human health hazard, and this volatized NH3 could cause water quality issues when it is later deposited on the surface of the Earth through precipitation. This could be extended to the application of ammonium and ammonia containing commercial fertilizers as well. To minimize concerns associated with air quality, it is recommended ammonia-containing fertilizers be incorporated upon application. There are agronomic and financial reasons for doing this as well as those associated with air quality. By incorporating these fertilizer sources, the amount of nitrogen lost from the soil system is reduced, thus, saving on the quantity of fertilizer purchases or allowing more land area to be fertilized with animal manure or biosolids. In addition to NH3, use of excessive nitrogen fertilizer may also result in the volatilization of nitrogen as di-nitrogen (N2) and nitrous oxide (N2O), depending on the soil and climate conditions. This usually only occurs when “micro” anaerobic conditions develop in wet soils or soils that received recent rainfall. While the loss of N2 is harmless to the environment, N2O is a considered to be a potent greenhouse gas.
Phosphorus
Environmental concerns with phosphorus focus on water quality, particularly surface water quality. Under normal conditions, phosphorus in the soil is an immobile plant nutrient and is tightly adsorbed to soil particles significantly reducing leaching movement through the soil profile. Therefore, under normal conditions if phosphorus is to reach surface water, it must be transported by the sediment load in runoff waters. If phosphorus does reach a stream or other body of surface water, it can lead to the accelerated eutrophication of the recipient water body. As previously discussed, eutrophication is the condition where a body of water has an enriched nutrient load but is limited by the available biological oxygen in the water. Algal species that proliferate in high phosphorus water include Anabaena, Ankstrodemus and Euglena. As these organisms die and are decomposed by other organisms, the available biological oxygen is significantly reduced causing adverse effects on other species of aquatic life. In general, phosphorus is considered the most limiting nutrient in surface waters. To reduce these adverse effects, proper application is needed.
The two main forms of phosphorus that can be transported from soils to surface waters are particulate phosphorus and dissolved phosphorus. Particulate phosphorus is the phosphorus that is bound to the soil particle and is transported only with eroded sediment. Dissolved phosphorus on the other hand, is the phosphorus that is found in the dissolved form in water. Only soils that have been built up with excessive phosphorus levels are able to contribute dissolved phosphorus in runoff or leachate water Thus, under normal circumstances, there is little risk of loss of dissolved phosphorus to runoff, and particulate phosphorus loss is prevented by conservation practices that reduce erosion and sediment transport. However, in soils containing excessive phosphorus concentrations, normal conservation practices that prevent transport of particulate phosphorus will do little to reduce transport of dissolved P. This is especially a problem because dissolved phosphorus is 100 percent bioavailable to aquatic life for causing eutrophication immediately upon deposition.
Soils that have excessive phosphorus concentrations that are able to supply high dissolved phosphorus concentrations to runoff are termed, “Legacy phosphorus soils”. The term “Legacy” is used because soils that are built up with high phosphorus levels will remain elevated in soil phosphorus concentrations for decades, unlike nitrogen, These soils will continue to contribute appreciable dissolved phosphorus to runoff during that time until the soil phosphorus concentrations decrease. The clear solution to this problem of reducing legacy phosphorus in soils is to “mine” phosphorus out of high phosphorus soils with crops that uptake high amounts of phosphorus (such as forages), while simultaneously ceasing all phosphorus applications. The plant matter must be harvested and removed from the site in order to reduce soil P. Studies have shown that depending on the initial soil phosphorus concentrations, “drawdown” of phosphorus can take 20 years or more before reducing soil phosphorus levels to below agronomic optimum. While this long-term solution is being implemented, phosphorus removal structures are an effective short-term solution to preventing the transport of dissolved phosphorus from legacy phosphorus soils to surface waters.
With regard to application of phosphorus sources, nearly all commercial phosphorus fertilizers are incorporated after broadcast application or banded below the seed. Again, reducing runoff and erosion will reduce environmental concerns related to P. As with nitrogen, the most effective way to do this is to follow good soil conservation practices. These include increasing water infiltration, reducing runoff by maintaining surface residues and using buffer strips at the edge of the field. These good conservation practices allow producers to maintain fertilizers, reduce soil loss and increase water stored in the soil profile.
Land application of animal manures, particularly poultry litter (high in phosphorus), and some biosolids are done by broadcasting the material on the soil surface. In many cases, these fertilizer materials are applied to forage crops, which eliminates their incorporation. When left on the surface in this manner, they may be subject to loss from the field in the runoff. To decrease the potential of phosphorus loss from these sources to surface waters, it may be necessary to apply using injection or knifing the material into the soil. Various technologies now exist that allow incorporation/injection of dairy, swine and poultry manure into forage systems with little disturbance of the surface. Basing manure application rates on crop phosphorus needs instead of nitrogen needs will slow down phosphorus build up in the soil and prevent them from becoming legacy phosphorus sources. Again, another method to reduce particulate phosphorus loss is to use a buffer strip at the edge of the field to reduce the amount of sediment and manure leaving the field.
Other Contaminants
With the decrease in suitable landfill sites for human waste and the increase in confined animal feeding operations, there has been a tremendous increase in the interest of land application of these materials. Land managers should view these materials as a valuable nutrient source and not a waste material. They contain many plant nutrients in addition to nitrogen and phosphorus, so operators who have them should use them to their maximum benefit. To date, no other constituents in these fertilizer sources have proven to be of major environmental concern when proper guidelines are followed. Each source has a different make-up due to ration formulation of materials in the municipal waste stream. Constituents which may need to be considered are copper in animal waste and heavy metals in biosolids. Heavy metal concentrations of biosolids must be monitored with materials above threshold levels needing to be landfilled. More information about biosolids land application is available from Oklahoma Department of Environmental Quality.
Environmental concerns due to the application of fertilizers can be drastically reduced by proper management of these resources. Regardless of fertilizer form, if the quantity applied is greater than what is required for the crop then the potential exists for negative environmental impacts. To minimize negative environmental impacts, there are a few simple practices land managers can use: add only the amount of fertilizer needed to meet plant requirements, use buffer strips and do not apply fertilizers too close to bodies of water, and use good soil conservation practices which minimize soil erosion and maximize water infiltration. A combination of these good management practices will greatly reduce the potential for adverse environmental impacts.
Chapter 8 - Land Application of Drilling Mud
Chad Penn
Drilling “mud” is a byproduct from drilling deep boreholes for oil and gas wells and also for relatively shallow boreholes in urban/suburban areas for installation of utilities such as sewer, electric and water. Drilling fluids are used during the drilling process for several purposes including bit lubrication and cooling, suspension and removal of cuttings, sealing of the borehole/formation and viscosity and pH control. As a result, the main ingredients added to the base drilling liquid often includes bentonite (a naturally occurring 2:1 soil mineral), polymers, soda ash, surfactants and “loss circulation materials” such as lignite (i.e. tiny particles of coal), rice hulls, cotton hulls, Styrofoam, etc.). Some oil and gas drilling fluids utilize barium sulfate. After the fluid has been used for drilling, a portion of it may be reclaimed/recycled for continued use on-site (Figure 8.1). At the point when the material is no longer able to be used in drilling, it often is then referred to as “drilling mud.” However, the drilling industry also will sometimes refer to the unused drilling fluid as “mud.” The drilling mud that is no longer able to be used for drilling must be disposed.
One of the most economical and sustainable methods of drilling mud disposal is land application to agricultural and range land soils. However, due to potential contaminants that may be found in certain types of drilling mud, it is extremely important that land application of mud be carefully executed in order to prevent permanent soil damage and negative environmental impacts. For organization purposes, this discussion will separate mud into two main categories: oil and gas drilling mud and horizontal directional drilling mud. The land application of drilling mud often is referred to as “soil farming,” or “land farming,” but technically that is a misuse of those terms. Land application involves a one-time application of drilling mud from a single well, while soil farming/land farming has multiple applications to the same site.
Figure 8.1. A typical schematic of drilling fluid/mud use during oil and gas drilling operations (from howstuffworks.com). Note the mud return line that allows removal of the drill cuttings from the borehole and recycling through the shale shaker.
Oil and Gas Drilling Mud
Land application companies will pay a landowner to receive oil and gas drilling mud on a volume basis; the total volume received will vary as a function of the size of the well being drilled. There are two main types of oil and gas drilling mud: water-base mud and oil-base mud. The main distinction between the two materials is the base solvent (i.e., liquid): WMB utilizes water while OBM utilizes diesel. In Oklahoma, WBM is used and produced more frequently compared to OBM. Water-base mud is mostly utilized while drilling the vertical portion of the borehole and the non-shale-like formations. The deeper portions and also the horizontal “curve” is where OBM typically is used. Landowners should expect a temporary yield decrease on fields that received drilling mud, assuming excessive volumes were not applied. Essentially, the landowner is compensated due to anticipated yield decrease. However, many landowners often experience no decrease in yield.
The land application of drilling mud is regulated by the Oklahoma Corporation Commission (OCC), which has certain site requirements for land application and also limits application rates based on total application of salts (total dissolved solids: TDS), chlorides, total petroleum-based hydrocarbons (TPH), and total solids. The rules for land application of WBM and OBM are specifically stated in the Oklahoma administrative code and register (www.oar.state.ok.us), Title 165 (165:10-7-19 and 165:10-7-26 for WBM and OBM, respectively).
Oil-base mud (OBM)
Oil-base mud is rich in TPH, and solids content ranges from 50 to 85 percent. Although not required by law, many land application companies will mix a “bulking agent” with OBM prior to land application. OBM is applied as a solid. The most common bulking agents are ag lime and gypsum. Depending on the bulking ratio and application rate, it is possible for ag lime to be applied at very high rates (more than 10 tons lime per acre). While this may be desirable in excessively acid soils, it is better to utilize gypsum as a bulking agent among neutral- and high-pH soils. It is especially advantageous to use gypsum as a bulking agent if the mud or soils contain appreciable sodium. However, some operators are utilizing new technology to further extract diesel from used OBM; the resulting dry solid material has no need for being mixed with a bulking agent.
The purpose of land application of OBM is to allow for native soil microorganisms to degrade and consume TPH, converting it to water and carbon dioxide. This process of microbial degradation is faster and more effective in soils where conditions are near neutral in pH, possess sufficient temperature and contain adequate nutrients and oxygen. While the OCC allows for application of 40,000 pounds TPH per acre, much of the research at OSU suggests TPH degradation rates suffer if TPH is applied greater than 10,000 to 15,000 lbs/acre. However, if an over-application of OBM does occur, tillage of the soil and addition of organic matter to dilute the OBM and introduce other soil microorganisms will typically improve degradation of TPH. While a portion of the TPH contains benzene, toluene, ethyl benzene and xylene (BTEX), leaching and incubation experiments at OSU have shown BTEX applied with OBM rapidly degrades and does not readily leach, even in sandy soils. Unlike WBM, OBM usually does not contain appreciable dissolved solids or sodium.
Figure 8.3. Oil-base mud shown in a staging cell constructed of gypsum, prior to mixing with gypsum and subsequent land application.
Water-base mud (WBM)
Depending on the geologic formation being drilled, WBM may contain extremely high levels of sodium and total dissolved solids, which are salts. Thus, certain regions of the state produce WBM that is more salty than other regions. Regarding soil quality and agronomic production, salt is even more important than TPH because salts do not degrade. For this reason, there is greater potential for soil damage with WBM than OBM. Water-base mud is applied mostly as a liquid; the goal of application is to dilute the salts over many acres to prevent negative impacts.
The main risk associated with receiving WBM is plant and soil damage at the surface due to salinity (excessive salts) and sodicity (excessive sodium).
See Chapter 3 for a discussion of soil salinity and sodicity. Even in scenarios where WBM is over applied causing damage to surface soils and plants, com- puter modelling scenarios have suggested that leaching of salts to groundwater is not likely to occur due to advection-dispersion processes.
Application of WBM is mostly limited by TDS. The OCC allows a maximum of 6,000 pounds TDS per acre, however, note that caution should be exercised since the OCC regulations do not consider the amount of sodium in the WBM, nor does it delineate between different soils, climate, or region. Recent research at OSU suggests that a TDS loading rate of 4,000 pounds TDS per acre is safer compared to 6,000 pounds TDS per acre. Regardless, rainfall is critical for the soil to recover from salt application. Rainfall allows the salts to leach out of the root zone. However, under excessively dry conditions, salt can potentially wick back up to the surface depending on soil texture and moisture.
Figure 8.4. Example land application of water-base mud (WBM).
Three main points of risk associated with receiving drilling mud applications are variation in climate, since rainfall is necessary for recovery, the type of plant, and the quality of the application company. Specifically, an application company that does not adequately characterize the mud or have the ability to closely control application rates is much more prone to causing serious soil damage compared to companies that follow OCC guidelines, characterize every load, and maintain suitable equipment that allows for more precise applications. If soils are not completely recovered from salt and sodium impacts, establishment of a new crop by sowing seed could suffer due to decreased germination rates. For this reason, there is less risk associated with application of WBM to perennial pasture and hay fields. For further details on oil and gas drilling mud, see the following:
- Rules, regulations and general risks with oil and gas drilling mud: OSU WREC-102
- OBM: Penn, C.J., A.H. Whitaker, and J.G. Warren. 2014. Surface application of oil-base drilling mud mixed with gypsum, limestone, and caliche. Agron. J. 106:1859-1866.
- WBM: OSU CR-2272
Figure 8.5. Example of a HDD drilling operation in an urban area.
Urban & Suburban Horizontal Directional Drilling (HDD) Mud
Drilling mud produced from installation of utilities is very different from oil and gas drilling mud.
HDD mud is produced from drilling through shallow soils and rock and possesses very little risk for causing soil damage upon land application.
A recent survey conducted by OSU of HDD mud from around the US showed that there was no limiting chemical constituent for land application. Instead, the most limiting factor for land application of HDD mud is usually the totals solids content. Essentially, the material is a soil slurry that takes on the chemical characteristics of the subsoil being drilled at the drilling site. Caution would only need to be exercised when the source of the HDD mud comes from a location that may have been previously contaminated in the subsoil; for example, sites that historically had industrial or mining activities that may have increased soil metals concentrations or salts. Application of HDD mud to Bermuda grass hay at OSU research plots showed no significant loss in biomass production, even when application rates were 100 tons solids per acre. In addition, HDD mud can be used to help establish grass on bare soils affected by recent construction; application of HDD mud at 20 tons solids per acre to bare soils with Bermuda grass seed improved germination compared to a control treatment that received no drilling mud. Application of 40, 60 and 80 tons solids per acre was not different from the control, while 100 tons per acre decreased germination compared to the control. For more information on HDD mud, please see OSU PSS-2916.
Chapter 9 - Long-term Fertility Research
Josh Bushong
Introduction
Few agronomic disciplines can compete with soil nutrient management and plant breeding concerning their impact on increased crop production in the world. However, both continue to be challenged considering our current global population of 7 billion, which is expected to exceed 9 billion by 2050. Future agronomic research efforts must result in technologies that increase yields per unit area and also must accomplish this feat with methods that are resource efficient and environmentally safe.
OSU has a rich history of conducting long-term soil fertility trials. The almost two-dozen continuous wheat, sorghum, and cotton soil fertility experiments have been instrumental in identifying efficient fertilizer sources as well as optimum application timings and rates of nitrogen, phosphorus, and potassium. Most of these experiments are still ongoing today; however, a few have been discontinued due to their lack of relevancy and/or cost. A complete list and brief description of all the long-term experiments conducted at OSU is maintained at nue.okstate.edu/Long_Term_Experiments.htm.
In previous editions of this handbook, this book chapter focused on summarizing the majority of the research that has been conducted by the OSU Soil Fertility Research Team. Some of these findings focused on the long-term experiments while others were from research projects that addressed current soil fertility and plant nutrient management issues important to the region. With now more than three decades of research having been conducted, summarizing all the projects could be a textbook in itself. A complete indexed list of all peer-reviewed, published research from experiments within this project is located at nue.okstate.edu/Index_Publications.htm. The purpose of this chapter in this edition of the Oklahoma Soil Fertility Handbook will be to highlight some of the recent findings from OSU’s historic, long-term soil fertility trials.
The Magruder Plots - Stillwater, OK
The Magruder Plots are the most prestigious research trial at OSU. They were established in 1892 and are the oldest continuous wheat soil fertility trials west of the Mississippi River. The data and discoveries garnered from these plots have been vast and only continue to grow. These plots, coupled with other long-term experiments, have demonstrated a marked decrease in soil organic matter over time in continuous cultivated wheat production systems (Figure 9.1). This discovery has led to researching and evaluating management practices capable of stabilizing soil organic matter levels. Despite the decrease in soil organic matter over the last 122 years, grain yields continue to increase with time, likely due to improved genetics (Girma et al. 2007a).
Figure 9.1. Decrease in soil organic matter for data gathered in selected years in the Magruder Plots, Stillwater, Oklahoma (adapted from Girma et al. 2007a).
Recent research conducted by soil microbiologists evaluated the effects of long-term soil fertility practices on nitrogen-fixing microorganisms. Collectively, these microbes fix approximately 100 to 180 million metric tons of nitrogen per year, which accounts for about 65 percent of nitrogen used in agricultural production. Many soil microbes possess the ability to fix atmospheric N2. One such group is called cyanobacteria. These nitrogen-fixing microbes are especially competitive and often thrive in soils receiving limited nitrogen fertilization. Following more than a century-long cultivation of winter wheat without fertilization, cyanobacteria in the check soil comprised about 2.6 percent of the bacterial community, whereas this value was 0.19 and 0.05 percent in soils supplemented with manure and NPK, respectively (Figure 9.2). This finding has significant implications for managing ecosystems, such as forestry, pasture, and rangeland, where chemical fertilization is limited. With the increasing demand and cost on energy, understanding means to promote proliferation of nitrogen-fixing microbes are also important for developing management strategies for sustainable agricultural production (Data courtesy of Dr. Shiping Deng and Ms. Sophia Li).
Experiment 502 - Lahoma, Oklahoma
Established in 1970 and located in the heart of Oklahoma’s wheat belt, this experiment has provided data for some of the most compelling breakthroughs in wheat nitrogen fertility. It was at this site, along with other long-term trials, in which results from soil cores, taken to a depth of ten feet clearly showed that no subsurface contamination of ammonium-nitrogen and nitrate-nitrogen was found when nitrogen was applied over the same area at the recommended rates for more than 20 years (Figure 9.3). This gave rise to the ‘Buffering Concept’ which explained why nitrate leaching from applied fertilizer in winter wheat was not expected under conventional practices and that excess nitrogen was actually lost via biological pathways.
Figure 9.2. Relative abundance of Cyanobacteria from different treatments in the Magruder Plots (Data courtesy of Dr. Shiping Deng and Ms. Sophia Li).
Figure 9.3. Soil ammonium-nitrogen and nitrate-nitrogen in pounds/acre/ profile increment as a function of nitrogen applied, following 20 years of annual applications in continuous winter wheat, Lahoma, Oklahoma.
It was at this experimental site that the concept of a response index, which compares the grain yield of a sufficiently fertilized plot to that of an unfertilized or insufficiently fertilized plot, was thoroughly documented. Johnson and Raun (2003) evaluated the grain yield response index values of this site over thirty years. They observed a wide range of levels of nitrogen fertilizer response over time that varied year-by-year (Figure 9.4). The overall conclusions of Johnson and Raun (2003) were that since response to nitrogen fertilizer is strongly dependent on supply of non-fertilizer nitrogen, any nitrogen fertilizer recommendations that include a reliable predictor of harvest response index should improve NUE in grain production. This work led to researchers at Oklahoma State and throughout the grain belt to develop ways to predict the harvest response index in-season for nitrogen fertilizer application recommendations. The most successful of these methods has been achieved with the use of NDVI readings of a sufficiently fertilized area (nitrogen-rich) and of an unfertilized or insufficiently fertilized area (Figure 9.5).
As previously stated, the nitrogen fertilizer response index has been found to be highly variable year to year. Using the data from this experimental site and other similar long-term nitrogen fertilizer trials, researchers from Oklahoma State also observed the grain yield or yield potential for each year varied greatly. Knowing that the level of nitrogen responsiveness and grain yield potential could affect the amount of nitrogen fertilizer required to achieve optimum yield and nitrogen use efficiency, Raun et al. (2011) began to evaluate the relationship between the two factors. The results they observed have become the cornerstone for nitrogen fertilizer recommendations in Oklahoma in that the nitrogen fertilizer response and grain yield potential were completely independent of one another (Figure 9.6). Because of their independence and that they each affect the demand for nitrogen fertilizer it is now proposed that estimates for both be combined when determining in-season nitrogen fertilizer rates.
Figure 9.4. Grain harvest response index for wheat to nitrogen fertilizer over time (Johnson and Raun, 2003).
Figure 9.5. Relationship between RINDVI and RIHarvest at Feekes growth Stage 5 over 22 site-years (adapted from Mullen et al., 2003).
Figure 9.6. Relationship between the response index and maximum yield of winter wheat at Lahoma, OK (502) and Stillwater, OK (222) (adapted from Raun et al., 2011).
Experiment 505 - Lahoma, Oklahoma
Various sources of nitrogen fertilizer are available to farmers in wheat production systems; however, few have been evaluated over a long period of time. In 1971, Experiment 505 at Lahoma, OK was initiated to compare different nitrogen sources and rates of nitrogen application on winter wheat grain yield. Few differences between nitrogen sources were observed in this experiment over time. Wheat grain yields increased significantly when nitrogen was applied at low annual nitrogen rates (30 to 60 pounds per acre), becoming greater with time. In recent years, split applied nitrogen had resulted in increased yields when compared using the same nitrogen source and total nitrogen rate (30-30 split versus 60 pounds nitrogen per acre applied preplant). Grain nitrogen continued to increase beyond the nitrogen rate required for maximum yield for most nitrogen sources. Much of what is described above has been documented in similar long-term trials or other trials in other parts of the country. One trend that was observed and thoroughly documented by Schroder et al. (2011) was grain yield began to decrease over time for the plots receiving the highest rates of nitrogen (120-240 pounds nitrogen per acre). Schoder et al. evaluated the soil pH of the plots in the trial and observed that for some nitrogen sources the soil pH was below 5.0 (Figure 9.7) and was dictating the crop yield response more than the applied nitrogen fertilizer. This phenomenon has become very common in much of the Oklahoma wheat belt where for years ammoniacal based fertilizers have been applied.
Figure 9.7. Soil pH vs. total nitrogen applied as (a) anhydrous ammonia or ammonium nitrate or (b) urea or sulfur coated urea for approximately 30 years. (Adapted From Schroder et al., 2007)
Experiment 222 - Stillwater, Oklahoma
Established in 1969, this experiment was initiated to evaluate the effects of nitrogen, phosphorus and potassium fertilization on the loamy prairie soils of Central Oklahoma. Most of the results of the major fertility breakthroughs described above in Experiment 502 at Lahoma, OK were mirrored in these experimental plots. Just like Experiment 502, no relationship between nitrogen responsiveness and grain yield potential was observed in these plots over time (Figure 9.6).
Because of the different combinations of fertilizer treatments, long-term fertility plots typically display vast differences in crop greenness and biomass throughout the growing season. This trait makes these trials ideal for developing and evaluating optical crop sensors. One such example from experiment 222 was from the work of Kanke et al. (2012). At the time, researchers from the Midwest were promoting sensors that utilized wavebands from the ‘red-edge’ spectrum. Kanke et al. (2012) evaluated these wavebands along with NDVI readings from the Greenseeker sensor and determined there was no difference between the two and reported no reason for upgrading current sensor to obtain this information.
Carbon sequestration has become an important issue in recent years, due to trends in global warming. One such sink for carbon has been the soil organic carbon fraction. Some studies have shown that increased nitrogen rates over time have increased the soil organic carbon fraction likely due to the increase in plant biomass that is returned to the soil after the growing season. To evaluate this theory, soil samples from this experimental site as well as experiment 502 at Lahoma, Oklahoma were collected recently and analyzed for soil organic carbon concentration. These results were then compared to archived soil samples from two decades ago. The results reported by Aula et al. (2016) were similar to those that have been reported by other researchers. Over time soil organic carbon had increased, especially in the highest nitrogen rate plots (Figure 9.8)
Experiment 406 & 407 - Altus, Oklahoma
Established in 1966, these two experiments have been utilized to evaluate the effects of nitrogen, phosphorus and potassium fertilization on irrigated and rain-fed winter wheat as well as the timing of nitrogen fertilizer application effects. A recent summarized analysis by Bushong et al. (2014) found if adequate soil moisture from irrigation or naturally occurring rainfall is available at planting, the application of sufficient levels of nitrogen fertilizer preplant is most beneficial to grain yield and overall water use efficiency. If plant available water is at average or below average levels, the timing of nitrogen fertilizer application is not as critical. The results for the response from phosphorusfertilization helped support the current recommendations in that grain yield responses were typically only observed with soil test phosphorus levels were below sufficient levels. A beneficial response to potassium fertilization was not observed, and should not have been expected with the high levels of soil test potassium for these sites coupled with the clays being of smectitic mineralogy.
Figure 9.8. Effect of nitrogen fertilization on soil organic carbon of surface soil (0-6 in) in Experiment 222 (E222), Stillwater, Oklahoma Experiment 502 (E502), Lahoma, Oklahoma in 1993, 2013 and 2014 (adapted from Aula et al., 2016).
When the original findings of Raun et al. (2011), which concluded that nitrogen responsiveness and grain yield potential were independent of one another, were contested by other soil nutrient management researchers, winter wheat data from these experimental sites along with the Magruder Plots were evaluated in a similar manner by Arnall et al. (2013). Much like the previous results observed, both sites displayed no relationship between the grain yield response index and grain yield potential (Figure 9.9). These results, along with the same results from long-term corn trials located in the midwestern United States, further supported the theory described above and that nitrogen fertilizer recommendations should be based on both crop nitrogen responsiveness and crop grain potential, separately.
Figure 9.9. Relationship between wheat grain yield and nitrogen response index (RI) for 143 years of site data from Altus and Stillwater, Oklahoma (adapted from Arnall et al., 2013).
Experiment 439 - Altus, Oklahoma
Established in 1972, this is the only long-term, irrigated cotton fertility trial associated with OSU. This trial evaluates the effects of nitrogen, phosphorus and potassium fertilization on cotton yield and lint quality. A summary of the results compiled by Girma et al. (2007b) showed that all three nutrients had some effect on lint yield, although most of the response was attributed to nitrogen and to some extent phosphorus. When the effects of fertilization on fiber length were evaluated, it was observed that excess nitrogen reduced nitrogen quality variables and the key to longer fibers was potassium fertilization, even with soil test potassium values well above sufficient levels.
Recently, research was reported that the amount of cotton seed required to produce one bale of cotton had decreased, leading researchers to hypothesize the nitrogen demand to produce the same yield of cotton should be less. Arnall and Boman (2012) used the data from Experiment 439 to evaluate the effect of nitrogen rate on lint yield and determined that the recommendation of 60 pounds nitrogen per bale should be reduced to 50 pounds nitrogen per bale.
Nitrogen and Phosphorus Response - Perkins, Oklahoma
This experiment was established in 1998 and evaluates the interactive effects of differing nitrogen and phosphorus rates on winter wheat grain yield and quality. The coarse texture that this trial is located on is ideal for potential responses to not only nitrogen, but also phosphorus. Girma et al. (2007c) analyzed seven years of data from this trial and observed differing levels of grain yield response to nitrogen. Their results showed temporal variability due to yield-limiting factors other than nitrogen can be a major factor controlling grain yield followed by nitrogen fertilizer. The results of this study also supported the current notion that average-based nitrogen recommendation should be avoided and producers need to shift to alternate strategies such as the use of nitrogen-rich strips. The application of phosphorus fertilizer was observed to only be beneficial in the first few years of this experiment. The experimental results support the approach of soil test phosphorus based recommendation of the amount that would equal the amount removed in harvested crops due to a lack of significance to phosphorus that was independent of years. Consequently, this demonstrates that variability in years, which is a function of weather related factors, did not have much effect on phosphorus use of the crop. In such cases an in-season crop demand for phosphorus might be satisfied with foliar phosphorus supplement.
Greenseeker sensor data has been collected from this site from its initial growing season. Because of the site’s coarse texture, this data set is unique from all the other long-term sites. It has been observed that grain yield potential prediction curves and response index prediction equations for this site are different than those of the loamier textured sites. This dataset currently is being evaluated with the hopes of developing yield and response index prediction algorithms specific for coarser textured surface soils.
Experiment 601 - Lake Carl Blackwell, Oklahoma
This experiment focuses on evaluating the long-term effects of different nitrogen rates applied preplant and mid-season on winter wheat. This experiment was established in 2001 and continues today. A recent analysis of the first 10 years of this experiment by Mohammed et al. (2013) reported that grain yield, grain protein, and nitrogen use efficiency were typically improved when nitrogen was split between two applications compared to being applied only once prior to planting. The data from this site also supports the theory of changes in nitrogen fertilizer response and grain yield potential from year-to-year by displaying large differences in grain yield were observed for the same nitrogen rates for different crop years.
Like most of the other nitrogen fertilizer response trials associated with the OSU, the plots for the experiment are extensively sensed throughout the growing season. This NDVI data is then used for developing new algorithms for nitrogen fertilizer recommendations or validating proposed or current nitrogen fertilizer recommendation algorithms.
Regional Wheat and Corn Nitrogen Response Trials - Various Locations
In the last decade, nitrogen fertilizer response trials for both winter wheat and corn have been established at several sites across Oklahoma. Though the design of these trials is simplistic with the only treatments being differing rates of nitrogen fertilizer applied preplant and/or mid-season, the data collected from these has been invaluable. These trials have allowed researchers to observe how differing factors, such as climate or soil type, can affect a crop’s nitrogen fertilizer response over time. Along with grain yield and quality data being collected, NDVI sensor data for these trials has been collected and systematically archived. This data has allowed researchers to develop and validate new algorithms and for predicting grain yield, grain protein, nitrogen fertilizer recommendations, and so many other items important to agronomic management decision making for cereal grain producers in Oklahoma.
Solie et al. (2012) utilized sensor and grain yield data from these regional trials along with other nitrogen response trials to develop a generalized algorithm for determining nitrogen rate recommendations for wheat and corn. With these datasets, Solie et al. (2012) developed a sigmoidal model for predicting grain yield (Figure 9.10) that allowed NDVI measurements collected in-season to apply nitrogen fertilizer with changing growth stages for both wheat and corn.
Using data from these regional wheat trials, Bushong et al. (2016) evaluated proposed and current nitrogen algorithms for determining nitrogen fertilizer recommendations. They observed all the sensor based nitrogen fertilizer recommendations provided more accurate predictions of the agronomic optimum nitrogen rates than current soil NO3 test methods, thus were more nitrogen use efficient. Similar results were observed when mid-season sensor-based nitrogen rates were compared to flat 50/50 split nitrogen rates. Throughout 20 site-years, on average the two methods yielded the same amount, however, the sensor based nitrogen rates were about half as much and returned on average $12 per acre for the producer (Table 9.1).
Figure 9.10. Sigmoid yield model with critical parameters developed by Solie et al. (2012).
Table 9.1. Sensor-based nitrogen rate compared to 50/50 split applications for winter wheat across 20 site years.
| Treatment | N Rate (lbs/acre) | Yield (bushels/acre) | Net Return ($/acre) |
|---|---|---|---|
| Split Application | 100 | 35 +/- 16 | $154 +/- $100 |
| SBNRC | 45 +/- 21 | 34 +/- 16 | $172 +/- $94 |
| Average Difference: $12 |
Fertilizer price (28-0-0) = $0.56 per pound nitrogen
Grain Price = $6.00 per bushel
Summary
What has been summarized above is just a small glimpse of the soil-nutrient-management work that has been conducted over the past few years at OSU. The tradition of the long-term trials will endure at Oklahoma State and will only continue to build robust datasets that future researchers will be able to use in order to make sound nutrient management decisions.
Acknowledgements
Drs. W.R. Raun, R.L. Westerman and B.B. Tucker, former faculty of the Plant and Soil Sciences Department of OSU for their foresight to establish, maintain and continue the long-term fertility studies that have made the above work and future work possible.
References
Arnall, B., and R. Boman. 2012. Cotton Yield Goal - Nitrogen Rate Recommendation. Oklahoma Cooperative Extension Service. PSS-2158-2.
Arnall, D.B., A.P. Mallarino, M.D. Ruark, G.E. Varvel, J.B. Solie, M.L. Stone, J.L. Mullock, R.K. Taylor, and W.R. Raun. 2013. Relationship between Grain Crop Yield Potential and Nitrogen Response. Agronomy Journal. 105:1335- 1344.
Aula, L., N. Macnack, P. Omara, J. Mullock, and W. Raun. 2016. Effect of Fertilizer Nitrogen (N) on Soil Organic Carbon, Total nitrogen and Soil pH in Long-Term Continuous Winter Wheat (Triticum Aestivum L.). Communications in Soil Science and Plant Analysis. http://dx.doi.org/10.1080/001036 24.2016.1147047.
Bushong, J.T., D.B. Arnall, and W.R. Raun. 2014. Effect of Preplant Irrigation, Nitrogen Fertilizer Application Timing, and Phosphorus and Potassium Fertilization on Winter Wheat Grain Yield and Water Use Efficiency. International Journal of Agronomy. http://dx.doi.org/10.1155/2014/312416.
Bushong, J.T., J.L. Mullock, E.C. Miller, W.R. Raun, and D.B. Arnall. 2016. Evaluation of Mid-Season Sensor Based Nitrogen Fertilizer Recommendations for Winter Wheat Using Different Estimates of Yield Potential. Precision Agriculture. http://dx.doi.org/10.1007/s11119-016-9431-3.
Girma, K., S.L. Holtz, D.B. Arnall, B.S. Tubana, and W.R. Raun. 2007a. The Magruder Plots: Untangling the Puzzle. Agronomy Journal. 99:1191-1198.
Girma, K., R.K. Teal, K.W. Freeman, R.K. Boman, and W.R. Raun. 2007b. Cot- ton Lint Yield and Quality as Affected by Applications of N, P, and K Fertilizers. The Journal of Cotton Science. 11:12-19.
Girma, K., K.W. Freeman, R. Teal, D.B. Arnall, B. Tubana, S. Holtz, and W.R.
Raun. 2007c. Analysis of yield variability in winter wheat due to temporal variability, and nitrogen and phosphorus fertilization. Archives of Agronomy and Soil Science.
Johnson, G.V., and W.R. Raun. 2003. Nitrogen Response Index as a Guide to Fertilizer Management. Journal of Plant Nutrition. 26:249-262.
Kanke, Y., W. Raun, J. Solie, M. Stone, and R. Taylor. 2012. Red Edge as a Potential Index for Detecting Differences in Plant Nitrogen Status in Winter Wheat. Journal of Plant Nutrition. 35:1526-1541.
Mohammed, Y.A., J. Kelly, B.K. Chim, E. Rutto, K. Waldshmidt, J. Mullock, G. Torres, K. Girma Desta, and W.R. Raun. 2013. Nitrogen Fertilizer Management for Improved Grain Quality and Yield in Winter Wheat in Oklahoma. Journal of Plant Nutrition. 36:749-761.
Mullen, R.W., K.W. Freeman, W.R. Raun, G.V. Johnson, M.L. Stone, and J.B. Solie. 2003. Identifying an In-season Response Index and the Potential to Increase Wheat Yield with Nitrogen. Agronomy Journal. 95:347-351.
Raun, W.R., J.B. Solie, and M.L. Stone. 2011. Independence of Yield Potential and Crop Nitrogen Response. Precision Agriculture. 12:508-518.
Schroder, J.L., H. Zhang, K. Desta, W.R. Raun, C.J. Penn, and M.E. Payton. 2011. Soil Acidification from Long-term Use of Nitrogen Fertilizers on Winter Wheat. Soil Science Society of America Journal. 75:957-964.
Solie, J.B., A.D. Monroe, W.R. Raun, and M.L. Stone. 2012. Generalized Algorithm for Variable-Rate Nitrogen Application in Cereal Grains. Agronomy Journal. 104:378-387.
Chapter 11 - Nitrogen-Rich Strips, GreenSeeker Sensor and Sensor-Based Nitrogen Rate Calculator
Joy Abit
The need to improve nitrogen recommendation strategies are more important today than ever as cost of commercial nitrogen fertilizer continue to rise steadily. Methods that increase nitrogen use efficiencies and farmers profitability are no longer simply commendable, but required. The Nitrogen-Rich Strip (nitrogen-rich strip) discussed in the next few pages along with the Sensor-Based Nitrogen Rate Calculator (SBNRC) can provide farmers with immediate improvement in N use efficiency and profitability.
The Nitrogen-Rich Strips
What are nitrogen-rich strips? The nitrogen-rich strip is an area in the field that has received a high rate of nitrogen than the rest of the field (Figure 1). The nitrogen-rich strip is used in conjunction with the GreenSeekerTM handheld sensor (discussed below) to determine the mid-season nitrogen rates. The rest of the field that receive the standard pre-plant rate is called the Farmer’s Practice.
Why use nitrogen-rich strip? The crop’s demand for fertilizer nitrogen and the amount of fertilizer nitrogen available in the soil greatly vary from year to year, even in fields where the same crop and the same amount of fertilizer rates are used every year. Why? Because the environment delivers a lot of free nitrogen in some years (warm, wet winters where lot of nitrogen is mineralized from soil organic matter, and nitrogen deposition in rainfall). A soil test provides an accurate determination of the amount of nitrogen available at the exact moment of sampling. However, pre-plant soil test does not provide all the necessary information for mid-season nitrogen rate decision. Producers need to have some knowledge of the crop yield potential and nitrogen mineralization to make fertility decisions.
Figure 11.1. Nitrogen-rich strip in a producer’s winter wheat field.
The nitrogen-rich strip is used to estimate the potential yield for that field and for that growing season. The amount of required nitrogen mid-season is gauged by comparing the farmer practice field area to that of the nitrogen-rich strip.
How and when to establish the nitrogen-rich strip? The strip should be at least 10 feet wide and 100 feet long. One strip is recommended in every field every year. For best results, the strip should be placed in a yield zone area or in each management area (such as different soil types or topography) of the field. It is best to change the location of the strip every year.
Zero nitrogen is not recommended unless soil test NO3 levels are high. Field should at least receive a starter. The amount of nitrogen applied is crop and region dependent. Table 11.1 shows the minimum amount of suggested nitrogen for both the nitrogen-rich strip and the rest of the field. To timely and effectively use the nitrogen-rich strip, the farmer’s practice rate should not exceed 50 percent of the yield goal recommended rate. Any source of nitrogen can be used for the nitrogen-Rich Strip.
Pre-plant application is the preferred timing, however for winter wheat and winter canola, application can be delayed up to 30 days after planting. An efficient way of applying nitrogen-rich strip is made by a double or triple pass of the applicator when pre-plant is being applied. Other methods of application can be reviewed in CR-227, Applying N-Rich Strips.
When to sense the nitrogen-rich strip? The nitrogen-rich strip is sensed when it becomes visible or prior to applying nitrogen mid-season. For winter wheat, sense prior to hollow stem, sensing and nitrogen application can take place after hollow stem but response to nitrogen decreases as crop nears flag leaf. Decisions about early nitrogen fertilization should be made when the nitrogen-rich strip appears to be better in condition than the rest of the field. When the nitrogen-rich strip looks the same as the rest of the field, take a sensor reading to determine if there is no true difference observed. If no difference is observed, continue checking the field on a regular basis.
Table 11.1. Minimum nitrogen rate recommendation for the Farmer Practice and N-Rich Strip.
| Crop | Minimum Total Nitrogen Level (Soil Test + Pre-plant) | |
|---|---|---|
| Farmer's Practice* (lbs N ac-1) | N-Rich Strip** (lbs N ac-1) | |
| Grain only wheat | 25 | 50 |
| Duel purpose wheat | 50 | 50 |
| Graze out wheat | 50 | 100 |
| Corn | 50 | 75 |
| Grain sorghum | 40 | 50 |
| Forage sorghum/corn silage | 50 | 100 |
| Bermudagrass | 50 | 100 |
*This value is equal to the residual NO3 level + pre-plant N+ at planting nitrogen, Under extremely dry conditions minimum total N level could be reduced.
**This is the rate of nitrogen above the farmer practice.
GreenSeekerTM Hand-held Sensor & Normalized Difference Vegetation Index
The GreenSeekerTM hand-held sensor is an easy-to-use optical sensor that instantly measure plant health and vigor in terms of NDVI readings. The sensor emits brief bursts of red and infrared light, and then measures the amount of each type of light that is reflected back from the plant.
How to use the GreenSeekerTM hand-held sensor. Point the sensor towards the ground then press and hold the trigger button located near the handle of the sensor. The sensor continues to sample the scanned area as long as the trigger remains engaged. When the trigger is released, the sensor displays the measured value in terms of an NDVI reading (ranging from 0.00 to 0.99) on its LCD display screen for 10 seconds.
What is NDVI? Normalized difference vegetation index (NDVI) is commonly used to measure plant health and vigor. One indicator of plant health is light absorption and reflectance. Healthy green plants absorb strongly wavelengths of visible (red, R) light and reflects wavelengths of near-infrared (NIR) light. Conversely, when plant are under stress, red band reflectance increases and near infrared band decreases. The strength of the detected light is a direct indicator of the health of the crop; the higher the reading, the healthier the plant. NDVI is a good biomass indicator and also implies total nitrogen content. The NDVI is typically calculated as follows:
NDVI = (NIR-R)/(NIR+R)
How to collect NDVI readings? Prior to applying fertilizer, collect NDVI readings from both nitrogen-rich strip and farmer practice plots at least 10 feet to 20 feet apart. Walk approximately 100 paces at the center of each plot. To ensure accuracy of your readings, hold the sensor 24 to 48 inches (60 to 120 centimeters) above the crop canopy when the trigger is pulled. Avoid sensing in areas that are unrepresentative of the remaining acres, including areas of poor crop stand.
Sensor-Based Nitrogen Rate Calculator
Sensor Based Nitrogen Rate Calculator enables farmers to estimate yield potential, obtain nitrogen fertilization rates, and decide if its practical and economical to apply nitrogen based on GreenSeeker sensor measurements, the response index, number of days where growing degree days are more than 0, agronomic maximum yield, expected grain price and fertilizer price.
What information do I need?
- NDVI readings from the nitrogen-Rich Strip and Farmer’s Practice fields.
- Planting date.
- Knowledge of the nearest Mesonet Station.
What should I do?
Step 1. Go to the Sensor-based nitrogen rate calculator webpage.
You can either google “NUE” or go to the website. Once at the website, go to the NUE tools and click on the “Sensor Based Nitrogen Rate Calculator” button (Figure 11.2a). This will bring a drop-down menu of options (Figure 11.2b. As of 2015, it has 32 options). Choose the crop of interest, for example, “Winter Wheat (US Grain Belt)”. This will bring you to the online calculator. Once you are on the online calculator webpage, there will be an input and output section. Scroll down to the bottom of the page then click “Within Oklahoma” button. This will allow the system to access the Oklahoma Mesonet site which gives the readings of temperature and growing degree days (GDD). These information are very important in the calculation of the N rate. That is also where the planting date becomes important.
A. B.
Figure 11.2. Sensor-based nitrogen rate calculator (A) and pull-down menu (B).
Step 2. Enter required data in the input section (Figure 11.3).
- Planting date. Date of wheat planting.
- Day prior to sensing. The day prior to sensing is necessary because this calculator relies on weather data from the Oklahoma Mesonet. Since Mesonet has not compiled the weather data for the current day (the day being sensed), enter the date prior to sensing.
- Location. Enter your location or choose the closest Mesonet site to the field of interest.
- NDVI Farmer Practice. This would be from an area in the field adjacent to where the N-rich strip was placed and that is representative of the rest of your field.
- NDVI Nitrogen-Rich Strip. This is the NDVI reading you got from your nitrogen-rich strip plot.
- NDVI values of both farmer’s practice and nitrogen-rich strip need to be collected within each and every field. Even if two adjacent fields differed in planting dates by only two days, the nitrogen recommendation is likely to be different.
- Producer estimate of max yield. Should be at least two to three times greater than the maximum yield for a field. The need for this input is to avoid fertilizing for unrealistic yields.
- Expected grain price and fertilizer cost. These numbers needs to be filled out but do not make a difference in the nitrogen rate that is recommended. It is something that you can use to decide whether or not applying fertilizer is economical.
Figure 11.3. Fill out required data in the input section.
Figure 11.4. The outputs.
What do these output values mean?
- Response index. This is the estimate of the responsiveness to applied nitrogen that a farmer is likely to encounter and that varies from year to year in the same field. The response index is essentially the NDVI of the nitrogen-rich strip divided by the NDVI of the farmer practice. If this is 1.33, it means that an increase of 33 percent can be achieved if fertilizer is ap- plied, but does not provide the nitrogen rate that should be applied.
- Days, GDD>0. Number of days the winter wheat has grown since it was planted. Growing degree days (GDD) is a way of assigning a heat value each day. The values are added together to give an estimate of the amount of seasonal growth the plants have achieved. GDD is computed as daily (Tmin + Tmax)/2 - 40 F. This basically determines the number of days were average temperatures were >40 F, or where growth was possible.
- Yield potential, YPO. Possible attainable yield without fertilizer.
- Yield potential, YPN. Possible attainable yield when fertilizer is applied.
- Yield potential is the estimated optimum yield that a farmer can ob- tain based on growing conditions from planting to sensing time. This is yield potential, not “yield” and essentially replaces “yield goals”. By dividing NDVI (an estimate of biomass) by GDD from planting to sensing, provide the biomass accumulated per number of days where growth was possible. Biomass produced per day and final grain yield has been shown to be highly correlated.
- Cumulative GDD. Value that is used for summer crops and winter canola. For winter wheat it is not important. Typically 80+ GDD>0 is needed for wheat and canola.
- N rate recommendation. Amount of N fertilizer that is needed to attain the YPN
- Gross return (no nitrogen fertilizer). The total expected rate of return with no nitrogen fertilizer
- Gross return (using nitrogen Rec). The total expected rate of return when the nitrogen rate recommendation is used.
Other Sources of Information
- www.nue.okstate.edu
- www.npk.okstate.edu
- www.osunpk.com
- CR-2277 – Applying Nitrogen-Rich Strips
- CR-2270 – Impact of Sensor-Based Nitrogen Management on Yield and Soil Quality
- PSS-2278 – Using the GreenSeekerTM Handheld Sensor and Sensor-based Nitrogen Rate Calculator
- PSS-2261 – Methods for Applying Topdress Nitrogen to Wheat
- PSS-2260 – The History of the GreenSeekerTM Sensor
- PSS-2258 – The Evolution of Reference Strips in Oklahoma
Chapter 12 - Laws and Acts Governing the Marketing of Fertilizer, Lime and Soil Amendments in Oklahoma
Bill Raun
The sale of fertilizer, agricultural lime and soil amendments is governed within Oklahoma by specific laws and acts. This legislation has been enacted by State Government to provide recognizable product standards and to protect unsuspecting consumers from marketing fraud. Provisions of the legislation are carried out by the State Department of Agriculture, Food and Forestry. Copies of each document may be obtained by request from:
Oklahoma State Department of Agriculture, Food and Forestry Consumer Protection Services
Division
2800 North Lincoln Blvd.
Oklahoma City, OK 73105-4298
Phone: 405-521-3864
Or online at: oda.state.ok.us/odaff-forms.htm
The laws and acts most important to soil fertility and soil management are:
- Oklahoma Fertilizer Act and Rules
- Oklahoma Soil Amendment Act and Rules
- Oklahoma Agricultural Liming Materials Act and Rules
This chapter includes excerpts from the laws and acts that should be of most interest to users of fertilizer, lime and soil amendments.
The Oklahoma Fertilizer Act and Rules
The Oklahoma fertilizer act contains several sections, each addressing a particular issue pertaining to fertilizer use in Oklahoma. These sections and significant excerpts relating to soil fertility and fertilizer use follow.
Statutes
§ 2-8-77.1. Short Title – Purpose - Preemption
-
- Sections 8-77.1 through 8-77.18 of this sub article shall be known and may be cited as the “Oklahoma Fertilizer Act.”
- The purpose of the Oklahoma Fertilizer Act is to provide assurances to the consumer that fertilizer products are properly identified, and that the quality represented by the manufacturer is accurate as well as for regulation of the storage, use, and application of fertilizer to protect the consumer and the environment.
- The Legislature hereby occupies and preempts the entire field of legislation in this state touching in any way the regulation and enforcement of the registration, labeling, sale, storage, transportation, distribution, notification of use, and agricultural use of fertilizer to the complete exclusion of any order, ordinance, or regulation by any municipality or other political subdivision of this state.
- No political subdivision shall regulate the registration, packaging, labeling, sale, storage, distribution, agricultural use or application of fertilizer. No political subdivision shall adopt or continue in effect local orders, ordinances, or regulations in this field, except for those relating to nonagricultural use or application or taxation relating to registration, packaging, labeling, sale, storage, distribution, use or application of fertilizers. Local legislation in violation of this section is void and unenforceable
§ 2-8-77.3. Definitions
Fertilizer material - Any substance containing one or more recognized plant nutrients which are used for its plant nutrient content and is designed for use or claimed to have value in promoting plant growth except unmanipulated animal and vegetable manures, marl, lime, limestone, and wood ashes.
Mixed fertilizer - Any combination or mixture of fertilizer materials.
Bulk fertilizer - A fertilizer distributed in a non-packaged form.
Custom blend - A fertilizer formulated according to specifications furnished by a final consumer.
Custom blender - A person who mixes or commingles commercial fertilizer into a custom blend and who
distributes such special blend. A custom blender shall not be required to register
each grade of fertilizer in the following circumstances:
-
-
-
- the custom blend is formulated according to specifications furnished by the ultimate consumer prior to mixing, and
- the custom blend is prepared by a lawn care or tree service company that mixes or commingles fertilizer and who applies the special blend for the ultimate consumer.
-
-
Brand - A term, design, or trademark used in connection with one or several grades of commercial fertilizer.
Label - The display of all written, printed, or graphic matter upon the immediate container, or a statement accompanying a fertilizer.
Labeling - All written, printed, or graphic matter, upon or accompanying any fertilizer, or advertisements, brochures, posters, or television and radio announcements used in promoting the sale of fertilizer.
Unmanipulated manures - Substances composed primarily of excreta, plant remains, or mixtures of these substances which have not been processed in any manner.
Manipulated manures - Substances composed primarily of animal excreta, plant remains or mixtures of these substances which have been processed by natural or mechanical drying or composting and no other chemicals have been added.
Grade - The percentage of total nitrogen, available phosphate, and soluble potash stated in whole numbers. Specialty fertilizers may be guaranteed in fractional units of less than one percent of total nitrogen, available phosphate, and soluble potash. Fertilizer materials, bone meal, manures, and similar materials may be guaranteed in fractional units.
Specialty fertilizer - A fertilizer sold in packages of less than thirty (30) pounds.
Distributor - Any person who distributes fertilizer.
Distribute - To import, consign, manufacture, blend, offer for sale, sell, barter,
commercially apply, or supply fertilizer in this state including, but not limited to, the delivery of bagged, labeled and registered fertilizer to a nonregis- trant that sells the fertilizer in this state.
Broker - A person who negotiates sales and purchases between a manufacturer, distributor, final consumer, or retailer of fertilizer.
Fertilizer dealer - Any person operating a business that is engaged in the distribution or sale of a fertilizer. The term fertilizer dealer shall not include an ultimate consumer who is engaged in the physical act of application of fertilizer or a retail store selling only bagged registered commercial fertilizer other than bagged ammonium nitrate.
§ 2-8-77.4. Manipulated Manures Excluded
Any person operating a business engaged in the distribution or sale of manipulated manures shall not be subject to provisions of Sections 8-77.5 through 8-77.7 of this title if manipulated manures offered for sale, sold, or distributed do not reflect by label any warrantees or guarantees of the contents of the manures other than the animal sources of the manures.
§ 2-8-77.5. Fees – License - Application
- The annual license fee for persons operating a business engaged in the distribution or sale of fertilizer shall be Fifty Dollars ($50.00) and expire on a date to be determined by the State Board of Agriculture.
- All fertilizer dealers shall obtain a license from the Board for each business location.
- An application for license shall include:
- The name and address of licensee; and
- The name and address of each business location in the state. The licensee shall inform the Board in writing of additional business locations established during the period of the license.
- No person, whose name appears on the label, shall distribute in this state fertilizer until it is registered with the Board by such person. An application for each brand and product name of each grade of fertilizer shall be made on a form furnished by the Board. Upon the approval of an application by the Board, a copy of the registration shall be furnished to the applicant. A distributor shall not be required to register any fertilizer which is already registered under the Oklahoma Fertilizer Act by another person, provided the label does not differ in any respect.
- Registrations for commercial fertilizer products sold in bulk quantities or packages of greater than thirty (30) pounds shall be permanent unless canceled by the registrant or the Board.
- 1. Registrations for specialty fertilizer products sold in packages of less than thirty
(30) pounds shall pay a one-hundred-dollar registration fee for each product.
2. Specialty fertilizer product registrations shall expire on June 30 of each year.
3. If the Board finds any specialty fertilizer products that have not been registered, a penalty of One Hundred Dollars ($100.00) per product will be assessed. The penalty shall be added to the registration fee and payment shall be made within thirty (30) days after receipt of notice. - A custom blender shall not be required to register each grade of fertilizer formulated according to specifications which are furnished by the final consumer prior to mixing, but shall be required to be licensed and shall be the guarantor of that custom blend.
- An application for registration shall include the following:
- The brand and grade;
- The guaranteed analysis;
- Name and address of the registrant;
- Net weight for packaged fertilizer; and
- Oklahoma fertilizer license number.
§ 2-8-77.6. Fertilizer Container Label Information
- Containers of fertilizer distributed in this state shall have placed on or affixed
to the container a label setting forth in clearly legible and conspicuous form the
following information:
-
Net weight;
-
Brand and grade;
-
Guaranteed analysis; and
-
Name and address of the registrant/licensee.
-
- In case of bulk shipments, this information in written or printed form shall accompany delivery.
- A fertilizer formulated according to specifications which are furnished by and for the final consumer prior to mixing shall be labeled to show the net weight, the guaranteed analysis, and the name and address of the distributor, registrant, or licensee.
§ 2-8-77.7. Inspection Fee – Semi Annual Statement – Exemptions - Penalty
-
-
- Each registrant distributing fertilizer in this state shall file with the State Board of Agriculture, not later than the last day of January and July of each year, a semiannual inspection fee report setting forth, under oath, the number of tons sold or distributed during the period and pay an inspection fee of One Dollar ($1.00) per ton of which fifty cents ($0.50) per ton shall be forwarded directly to a special Soil Fertility Research Account in the Department of Plant and Soil Sciences of the Division of Agricultural Sciences and Natural Resources at OSU for the purpose of conducting soil fertility research and extension involving efficient fertilizer use for agronomic crops and forages and groundwater and surface water protection from plant food nutrients. OSU shall present an annual report to the Agriculture Committees of the Legislature on the use of the special Soil Fertility Research Account Fund.
-
- Each registrant distributing commercial fertilizer in this state shall file with the
State Board of Agriculture not later than the last day of January and July of each
year, a semiannual tonnage report stating under oath:
-
- The number of net tons of fertilizer distributed during the preceding six (6) calendar months;
- The amount in tons of each grade of fertilizer distributed during the preceding six (6) calendar months; and
- Whether the fertilizer was distributed in bag, bulk, or liquid.
-
- If no fertilizer was sold or distributed in this state for the semiannual period, the registrant shall submit a statement reflecting that information and shall remit a minimum fee of Ten Dollars ($10.00). If the inspection fee and tonnage report are not filed and the payment of the inspection fee is not made within thirty (30) days after the end of the specified filing period, a collection fee of ten percent (10%) of the inspection fee due or a minimum Ten Dollars ($10.00), shall be assessed and added to the amount due.
- Sales or exchanges between importers, manufacturers, distributors, registrants, or licensees are exempt.
- When more than one person is involved in the distribution of a fertilizer, the last person who has the fertilizer registered and who distributed the fertilizer to a nonregistrant dealer or consumer is responsible for reporting the tonnage and paying the inspection fee, unless the report and payment is made by a prior distributor or manufacturer of the fertilizer.
- If the Board finds any deficient inspection fees due as a result of an audit of the records of any person subject to the provisions of the Oklahoma Fertilizer Act, the Board shall assess a penalty fee of ten percent (10%) of the amount due, with a maximum not to exceed Two Thousand Dollars ($2,000.00) or a minimum of One Hundred Dollars ($100.00) whichever is greater. The audit penalty shall be added to the deficient inspection fees due and payment shall be made within thirty (30) days of notice of the deficiency.
- No information furnished to the Board under this section shall be disclosed in a way which divulges proprietary information about the operation of any person.
- Each registrant, distributor, or manufacturer shall keep accurate records of the tonnage of fertilizer distributed in this state.
§ 2-8-77.9. Sampling And Analysis Methods
- The methods of sampling and analysis shall be those adopted by the Association of Official Analytical Chemists. In cases not covered by these methods, or in cases where methods are available in which improved applicability has been demonstrated, the State Board of Agriculture may adopt appropriate methods from other sources.
- The Board, in determining for administrative purposes, whether any fertilizer is deficient in plant food, shall be guided solely by the official sample as defined in Section 8-77.3 of Title 2 of the Oklahoma Statutes and obtained and analyzed as provided for in subsection A of this section.
- Official samples establishing a penalty for nutrient deficiency shall be retained for a minimum of ninety (90) days from issuance of a deficiency report.
§ 2-8-77.10. Penalties
- A payment of two (2) times the value of the deficiency or deficiencies shall be assessed:
- If the analysis shows that a fertilizer is deficient in one of its guaranteed primary plant nutrients beyond the investigational allowances and compensations as established by rules; or
- If the overall commercial value of the fertilizer is below the level established by rule, a penalty payment of two (2) times the value of the deficiency or deficiencies shall be assessed.
- When a fertilizer is subject to a penalty payment under subsection A of this section, the larger penalty payment shall apply.
- All penalty payments assessed under this subsection A of this section shall be paid by the registrant or licensee to the consumer of the lot of fertilizer represented by the sample analyzed within thirty (30) days after the date of notice. Copies of consumer refund receipts shall be forwarded to the State Board of Agriculture. If a consumer cannot be found, the penalty shall be paid and deposited in the State Department of Agriculture Revolving Fund.
- A deficiency in an official sample of mixed fertilizer resulting from non-uniformity is not distinguishable from a deficiency due to actual plant nutrient shortage and is properly subject to official action.
§ 2-8-77.11. Determining Commercial Value For Purpose Of Assessing Penalty
For the purpose of determining the commercial value to be applied under the provisions of Section 8-77.10 of Title 2 of the Oklahoma Statutes, the State Board of Agriculture or its agent shall determine the values per unit of nitrogen, available phosphate, and soluble potash in fertilizers in this state. The value determined shall be used in assessing penalty payments.
§ 2-8-77.12. Misbranded Fertilizer
No person shall distribute misbranded fertilizer. A fertilizer shall be misbranded if:
- Its labeling is false or misleading;
- It is distributed under the name of another fertilizer product; or
- It is not labeled as required in Section 8-77.5 of Title 2 of the Oklahoma Statutes and rules promulgated by the State Board of Agriculture.
§ 2-8-77.14. Authority To Publish Information
This section provides for the publication of test results for the analysis of fertilizers as compared to their guaranteed analysis and for the publication of the sale and distribution of fertilizer in the state.
§ 2-8-77.15. Contamination Of Ground Water – Preventive Measures - Jurisdiction
This section prohibits fertilizer discharges.
§ 2-8-77.16. Seizure Of Fertilizer
This section provides the Board authority to take appropriate action in the event fertilizer sales are in violation of this act.
§ 2-8-77.17. Prosecutorial Discretion - Minor Violations
This section allows for discretionary enforcement action for minor violations by utilizing notice of violations and warnings.
§ 2-8-77.18. Sales And Exchanges Of Licensed Brands Among Importers, Manufactures, Or Manipulators
Allows free exchange of materials among members of the industry.
Rules
35:30-29-22. General
-
-
- Guarantee requirements. Other plant nutrients when mentioned in any form or manner
shall be registered and shall be guaranteed. Guarantees shall be made on the elemental
basis. Sources of the elements guaranteed and proof of availability shall be provided
to the Board upon request. Except guarantees for those water soluble nutrients labeled
for ready to use foliar fertilizer, ready to use specifically liquid fertilizer, hydroponic,
or continuous liquid feed programs and guarantees for potting soils, the minimum percentages
that shall be accepted for registration are as follows:
(1) Calcium (Ca) - 1.0000%
(2) Magnesium (Mg) - 0.5000%
(3) Sulfur (S) - 1.0000%(4) Boron (B) - 0.0200%
(5) Chlorine (Cl) - 0.1000%
(6) Cobalt (Co) - 0.0005%
(7) Copper (Cu) - 0.0500%
(8) Iron (Fe) - 0.1000%
(9) Manganese (Mn) - 0.0500%
(10) Molybdenum (Mo) - 0.0005%
(11) Sodium (Na) - 0.1000%
(12) Zinc (Zn) - 0.0500%
- Guarantee requirements. Other plant nutrients when mentioned in any form or manner
shall be registered and shall be guaranteed. Guarantees shall be made on the elemental
basis. Sources of the elements guaranteed and proof of availability shall be provided
to the Board upon request. Except guarantees for those water soluble nutrients labeled
for ready to use foliar fertilizer, ready to use specifically liquid fertilizer, hydroponic,
or continuous liquid feed programs and guarantees for potting soils, the minimum percentages
that shall be accepted for registration are as follows:
-
- Guarantees for plant nutrients. Only guarantees or claims for the above listed plant nutrients recognized by AAPFCO shall be accepted. Proposed labels and directions for the use of the fertilizer shall be furnished with the application for registration upon request. Any of the above listed elements that are guaranteed shall appear in the order listed and shall immediately follow guarantees for the primary nutrients of nitrogen, phosphate, and potash.
- Warning or caution statement. A warning or caution statement may be re- quired for any product which contains a nutrient in water soluble form when there is evidence that the micro-nutrient is present in excess of a guaranteed percentage that may be harmful to certain crops or where there are unusual environmental conditions.
- Examples of warning or caution statements:
(1) Directions: Apply this fertilizer at a maximum rate of (number of pounds) per acre for (name of crop). (2) CAUTION: Do not use on other crops. The (name of micro-nutrient) may cause injury to them. (3) CAUTION: Apply this fertilizer at a maximum rate of (number of pounds) per acre for (name of crop). Do not use on other crops; the (name of micro-nutrient) may cause serious injury to them. (4) WARNING: This fertilizer carries added (name of micro-nutrient) and is intended for use only on (name of crop). Its use on any other crops or under conditions other than those recommended may result in serious injury to the crops. (5) CAUTION: This fertilizer is to be used only on soil which responds to (name of micro-nutrient). Crops high in (micro-nutrient) are toxic to grazing animals (ruminants). (6) CAUTION: (Name of micro-nutrient) is recommended for all crops where (name of micro-nutrient) may be deficient; however, excessive application to susceptible crops may cause damage. - Fertilizer labels. The following information, in the format presented in Appendix
A of this Chapter, is the minimum required for all fertilizer labels. For packaged
products, this information shall either (1) appear on the front or back of the package,
(2) occupy at least the upper-third of a side of the package, or (3) be printed on
a tag and attached to the package. This information shall be in a readable and conspicuous
form. For bulk products, this same information in written or printed form shall accompany
delivery and be supplied to the purchaser at time of delivery. (1) Net weight (2)
Brand (3) Grade (4) Guaranteed Analysis
EXAMPLES FROM APPENDIX A:
Total Nitrogen (N)* ............................................................______% ______% Ammoniacal Nitrogen
______% Nitrate Nitrogen
______% Water Insoluble Nitrogen
______% Urea Nitrogen
______% (Other recognized and determinable forms of N)
Available Phosphate (P2O5) ................................................______% Soluble Potash (K2O) .........................................................______% (Other nutrients, elemental basis)..........................................______% *If chemical forms of any nutrients are claimed or required, the chemical forms shall be shown. (5) Sources of nutrients shall be listed below the completed guaranteed analysis statement. (6) Name and address of registrant or licensee.
(7) Directions for use for fertilizer to the end user shall follow the guidelines established by the Association of American Plant Food Control Officials. - Plant nutrients. When a plant nutrient is broken down into the component forms, the percentage for each component shall be shown before the name of the form as illustrated in Appendix B of this Chapter.
EXAMPLES FROM APPENDIX B:
Total Nitrogen (N) ................................................ ________%
________% Ammoniacal Nitrogen ________% Nitrate Nitrogen
Magnesium (Mg) .................................................. ________% ________% Water Soluble Magnesium (Mg)
Sulfur (S) ........................................................... ________% _______% Free Sulfur (S)
_______% Combined Sulfur (S)
Iron (Fe) ............................................................ ________%
_______% Chelated Iron (Fe)
Manganese (Mn) ................................................. ________%
_______% Water Soluble Manganese (Mn)
G. Slowly released plant nutrients.
(1) No fertilizer label shall bear a statement that implies that certain plant nutrients contained in a fertilizer are released slowly over a period of time, unless the slow release components are identified and guaranteed at a level of at least 15% of the total guarantee for that nutrient.
(2) Types of products with slow release properties recognized are:
(1) Water insoluble, such as natural organics, ureaform materials, urea-formaldehyde
products, isobutylidene diurea, oxamide, etc.,
(2) Coated slow release, such as sulfur coated urea and other encapsulated soluble
fertilizer,
(3) Occluded slow release, where fertilizer or fertilizer materials are mixed with
waxes, resins, or other inert materials an formed into particles, and
(4) Products containing water soluble nitrogen such as ureaform materials, urea formaldehyde products, methylenediurea (MDU), dimethylenetriurea (DMTU), dicyanodiamide (DCD), etc. The terms “water insoluble,” “coated slow release,” “slow release,” “controlled release,” “slowly available water soluble” and “occluded slow release” are accepted as descriptive of these products, provided the manufacturer can show a testing program substantiating the claim (testing under guidance of Experiment Station personnel or a recognized reputable researcher acceptable to the Board). A laboratory procedure, acceptable to the Board for evaluating the release characteristics of the product(s) shall also be provided by the manufacturer.
(3) Until more appropriate methods are developed, AOAC International Method 970.04 (15th Edition) is to be used to confirm the coated slow release and occluded slow release nutrients and others whose slow release characteristics depend on particle size. AOAC International Method 945.01 (15th Edition) shall be used to determine the water insoluble nitrogen of organic materials.
H. Definitions. Except as the Board designates in specific cases, the names and definitions for commercial fertilizer shall be those adopted by the Association of American Plant Food Control Officials.
I. Percentages. The term of “percentage” by symbol or word, when used on a fertilizer
label shall represent only the amount of individual plant nutrients in relation to
the total product by weight.
J. Penalties. When the combined commercial value for total nitrogen, available phosphoric
acid or phosphate P2O5, and soluble potash is found to be 4% or more deficient from the guarantee, or when
any one of the above is found to be 10% deficient from the guarantee, the penalty
assessed the manufacturer, or custom blender shall be twice the commercial value of
the nutrient deficiency. Penalties shall be assessed in accordance with the AAPFCO
formula: a 4% penalty is calculated at twice the value of the deficiency times total
tons (i.e., 5 tons of 34-0-0 found to be 30.97-0-0 is 2 x $12.12 x 5); a 10% penalty
is calculated at twice the units deficient times the value per unit times total tons
(i.e., 5 tons of 27-13-13 found to be 23.26-13-13 is 2 x 3.76 x commercial value x
5). When a fertilizer is subject to a penalty payment under both 4% and 10%, the larger
penalty shall be assessed.
(1) A deficiency in an official sample of mixed fertilizer resulting from non-uniformity
is not distinguishable from a deficiency due to actual plant nutrient shortage and
is properly subject of official action.
(2) The commercial values of fertilizer shall be established by the Board for calculating
penalties.
(3) Penalty assessment refunds shall be documented by receipts signed by the consumer
acknowledging the refund or credit, and shall be furnished to the Board within forty-five
(45) days after receiving notice of the penalty assessed. If the consumer(s) cannot
be found, the penalty (or amount not refunded) shall be paid to the Board within forty-five
(45) days after receiving notice of the penalty assessed.
K. Organic nitrogen. If an amount of nitrogen is designated as organic, then the water insoluble nitrogen or the slow release nitrogen guarantee shall not be less than 60% of the nitrogen so designated. Coated urea shall not be included in meeting the 60% requirement.
L. Discharges. For the purpose of protecting surface and groundwater, any discharge
of two hundred (200) pounds of dry or fifty-five (55) gallons or more of liquid fertilizer
shall be reported (telephone or fax) to the Board or its authorized agent within 24
hours if discharged outside the loading, transfer or application area.
M. Accidental discharge response plan for dry, liquid, and anhydrous ammonia. The
operator of a commercial storage facility shall prepare a written “Discharge response
plan” for the storage facility. The plan shall include:
(1) The identity and telephone number of the persons or agencies who are to be contacted
in the event of a discharge, including persons responsible for the stored fertilizer;
and,
(2) For each bulk fertilizer stored at the facility, a complete copy of the storage
container labeling required by these rules and the labeling required under Oklahoma
Fertilizer Law to accompany sale of the fertilizer; and,
(3) An identification, by location, of every storage container located at the storage
facility, and the type of bulk fertilizer stored in each storage container; and,
(4) For each type of bulk fertilizer stored at the facility, the procedures to be
used in controlling and recovering, or otherwise responding to a discharge; and,
(5) Procedures to be followed in using or disposing of a recovered discharge.
N. Availability. A copy of the discharge response plan shall be kept readily available
at the storage facility and at the nearest local office from which the storage facility
is administered.
O. Community awareness. The operator of a commercial storage facility shall inform
the local fire and police departments, and the appropriate state environmental agency,
of the existence of the plan and shall provide a current copy of the plan to the local
fire and police departments and the appropriate state environmental agency.
35:30-29-23. Heavy Metals
Fertilizers stating guaranteed amounts of phosphates or micronutrients shall be considered adulterated if the fertilizers contain metals in amounts greater than the levels of metals established by the Association of American Plant Food Control Officials in the SUIP 25 guide.
Oklahoma Soil amendment Act and Rules
Statutes
This legislation has many of the same provisions as the Oklahoma Fertilizer Law and the Oklahoma Liming Materials Act. Additional, relevant provisions include the following.
§ 2-8-85.3. Definitions.
Soil Amendment - Includes any substance which is intended to improve the physical, chemical or other characteristics of the soil, horticultural growing media, or any natural or synthetic substance applied to plants or seeds that is intended to improve crop production, germination, growth, yield, product quality, reproduction, flavor or other desirable characteristics of plants except the following: commercial fertilizers, agricultural liming materials, agricultural gypsum, unmanipulated animal manures, unmanipulated vegetable manures and pesticides; provided that commercial fertilizer shall be included if it is represented to contain, as an active ingredient, a substance other than a recognized plant food element or is represented as promoting plant growth by other than supplying a recognized plant food element.
Labeling - All written, printed or graphic matter upon or accompanying any soil amendment, and all advertisements, brochures, posters, television or radio announcements used in promoting the sale of such soil amendments.
Active Ingredient or Soil Amending Ingredient
- The ingredient or ingredients which affect the physical, chemical or other characteristics of the soil and thereby improve soil conditions.
- any natural or synthetic substance when applied to plants or seeds that is intended
to improve crop production, germination, growth, yield, product quality, reproduction,
flavor, or other desirable characteristics of plants
Inert Ingredient or Other Ingredient - The ingredients with no beneficial effect that are present in the product
Misbranded - Means and shall apply if:
- any soil amendment bears a label that is false or misleading in any particular,
- any soil amendment is distributed under the name of another soil amendment,
- any material is represented as a soil amendment or is represented as containing a soil amendment, unless such soil amendment conforms to the definition of identity, if any, prescribed by regulation,
- the active ingredient in any soil amendment is not shown in the approved ingredient form, or
- the labeling on any soil amendment is false or misleading in any particular.
Adulterated means and shall apply to any soil amendment if:
a. it contains any deleterious or harmful agent in sufficient amount to render it
injurious to beneficial plants, animals, or aquatic life when applied in accordance
with the directions for use shown on the label; or if adequate warning statements
and directions for use, necessary to protect plants, animals, or aquatic life are
not shown on the label,
b. its composition falls below purported labeling requirements, or
c. it contains noxious weed seed
§ 2-8-85.4. Labeling.
A. Each container of a soil amendment shall be labeled on the face or display side in a readable and conspicuous form to show the following information:
1) The net weight of the contents;
2) The name of the product;
3) The guaranteed analysis;
4) A statement as to the purpose of the product;
5) Adequate directions for use; and
6) The name and address of the registrant.
B. Bulk lots shall be labeled by attaching a copy of the label to the invoice that shall be furnished to the purchaser.
C. The State Board of Agriculture may require proof of claims made for any soil amendment.
If no claims are made the Board may require proof of usefulness and value of the soil
amendment. For evidence of proof the Board may rely on experimental data, evaluations,
or advice supplied from sources including but not limited to the Director of the Agricultural
Experiment Station. The experimental design shall be related to Oklahoma conditions
for which the product is intended. The Board may accept or reject other sources of
proof as additional evidence in evaluating soil amendments.
D. No soil amending ingredient may be listed or guaranteed on the labels or labeling of soil amendments without Board approval.
E. The Board may allow a soil amending ingredient to be listed or guaranteed on the label or labeling if satisfactory supportive data is provided the Board to substantiate the value and usefulness of the soil amending ingredients. The Board may rely on outside sources including but not limited to the Director of the Agricultural Experiment Station for assistance in evaluating the data submitted.
F. If the Board approves the listing of guarantee of a soil amending ingredient it shall be subject to inspection and analysis.
G. The Board may prescribe methods and procedures of inspection and analysis of the soil amending ingredient. The Board may stipulate, by rule, the quantities of the soil amending ingredient or soil amending ingredients required in soil amendments.
§ 2-8-85.5. Registration — Fee.
- Each soil amendment product shall be registered with the State Board of Agriculture before it is distributed in this state. Application for registration shall be submitted to the Board, on a form, showing the information required on the label, as provided in Section 8-85.4 of this title and rules promulgated pursuant thereto, except net weight of product.
- The registration fee shall be One Hundred Dollars ($100.00) for each product.
- All registrations shall expire on December 31 of the year for which the soil amendment product is registered.
- The applicant shall submit with the application for registration a copy of the label and a copy of all advertisements, brochures, posters, and television and radio announcements to be used in promoting the sale of the soil amendment.
- If the Board finds any soil amendment product that has not been registered, the registration was falsely submitted, or the registration was late, the Board may establish and assess a penalty. The penalty shall be be assessed per product and added to the registration fee and payment shall be made within thirty (30) days after receipt of notice.
§ 2-8-85.8. Violations.
It shall be a violation of the Soil Amendment Act for any person:
- To distribute a soil amendment that is not registered with the State Board of Agriculture;
- To distribute a soil amendment that is not labeled;
- To distribute a soil amendment that is misbranded;
- To distribute a soil amendment that is adulterated;
- To fail to comply with a stop sale, stop use, or removal order; or
- To violate any other provision of the Soil Amendment Act.
Rules
35:30-30-1. Definitions
Biosolid – A primary organic solid material produced by wastewater treatment processes that can be beneficially recycled for its plant nutrient content and soil amending characteristics, as regulated pursuant to 40 CFR 503, as amended.
Custom Media - A horticultural growing medium that is prepared to exact specifications of the person utilizing the medium.
Horticultural Growing Media - Any substance or mixture of substances promoted as or is intended to function as a growing medium for the managed growth of horticultural crops in containers and shall be considered a soil amendment for the purposes of this chapter.
Microbial Based - A biological substance or mixture of substances distributed to be applied to the soil, plants,or seeds for corrective soil purposes; intended to improve germination, growth yield, product quality, reproduction,flavor, or other desirable characteristics of plants; or intended to produce any chemical, biochemical, biological, or physical change in the soil.
35:30-30-2. Registration and fees
- Each soil amendment product shall be registered with the Board prior to distribution on a registration document supplied by the Board.
- All registrations expire on December 31st of the year registered.
- No product name shall be registered that misrepresents the product’s primary component or component formulation.
- Each product name shall refer to a specific formulation; different product names may refer to the same specific formulation. Products for which formulations change or are modified beyond the ranges reported in the registration document shall either be reregistered with a name that distinguishes them from the previous formulation, or production and distribution of the previous formulation shall cease.
- Reregistered products shall be accompanied by a new registration document for that formulation.
- Each product registration document shall be accompanied by a label or facsimile of a label for that product as named. If the same product is sold in more than one size, only one label sample shall be submitted.
- The Board shall not issue and may revoke any soil amendment registration if the Board determines the registration is for the primary purpose of disposal of the product or substance.
- The registration fee shall be One Hundred Dollars ($100.00) for each product.
- If the Board finds that any soil amendment product is not registered, a penalty of One Hundred Dollars ($100.00) per product shall be assessed. The penalty shall be added to the registration fee and payment shall be made within thirty (30) days after receipt of notice.
35:30-30-3. Contents of the label
(a) Label information may be printed on the primary or secondary display panel on
the bag containing the product, printed on a sticker placed on the bag, printed on
a flyer or tag attached to the bag, or in the case of bulk bags or bulk, any of the
above or printed on a fact sheet accompanying the shipment.
(b) The Board shall require each label to contain the following minimum information. Additional information of an instructional or explanatory nature may be provided at the discretion of the registrant.
(1) The product name as registered.
(2) The quantity of the product in quarts, cubic feet, yards, or metric equivalents
or the weight of the product in ounces, pounds, tons or metric weights or the fluid
measure in fluid oz, quarts or gallons or metric equivalents as determined by the
dominant method of sale by the industry and as registered.
(3) The guaranteed analysis for inorganic based soil amendments shall include the name and the percentage of each active ingredient, and the percentage of inert ingredients.
(4) The guaranteed analysis for microbiological based soil amendments intended as an inoculum shall include the expiration date, state the number and kind of viable organisms per milliliter, or, if the product is other than liquid, state the number and kind of viable organisms per gram. If the product is not intended as an inoculum, then the product label shall state that the product is not a viable culture.
(5) In lieu of a guaranteed analysis for organic based soil amendments an ingredient list shall show all components whether organic or inorganic. Components shall be listed in order of decreasing volume, if they comprise at least three percent (3%) or more of the total volume of the product. Components shall be described as follows:
(A) Bark products shall be described as raw, aged, processed, or composted. Bark shall also be specified as pine or softwood (meaning Gymnosperm), or hardwood (not Gymnosperm), and may include no more than fifteen per cent (15%) wood by volume.
(B) Peat products shall be described in accordance with ASTM standards as to whether they are sphagnum, hypnum, reed-sedge, humus, or other peat. Wood products shall be described as raw, aged, processed, reprocessed or composted.
(D) Readily degradable organic substances shall be listed and described as raw, aged, processed or composted.
(E) The base material for any other composted product shall be described as listed.
(F) Mulches shall be described as listed in the components.
(G) Manures shall be described as listed in the components.
(6) Application rates and intended use statements such as general recommendations
for product use. If cautionary warnings of uses not recommended are made, they should
be stated in this section of the label.
(7) An address where further product information may be obtained, and a telephone
number available during normal business hours for further product information.
(8) For products intended for use by commercial growers, the date of manufacture,
or the month and year of manufacture, stated at any location on the bag. If the date
or month and year of manufacture is coded, sufficient information must be provided
to determine the date or month and year of manufacture from the code.
(9) The Board may require a registrant to include a warning or caution statement to ensure safety.
35:30-30-6. Exemptions
(a) Distribution of horticultural growing media planted with live plant material is exempt from the labeling and registration requirements.
(b) Distribution of custom media is exempt from registration requirements imposed provided it is prepared for a single end user.
(c) Distribution of a soil amendment that is registered pursuant to the Oklahoma Fertilizer Act may be exempt from the registration requirement, but shall not be exempt from any requirements other than registration.
Oklahoma Agricultural Liming Materials Act and Rules
Statutes
In addition to the provisions identified by the Oklahoma Fertilizer Law and the Oklahoma Soil Amendment Act, the Oklahoma Agricultural Liming Materials Act provides for the following specifics relevant to liming materials.
§ 2-8-80.2. Definitions:
Agricultural Liming Material - A product whose calcium and magnesium compounds are capable of neutralizing soil acidity.
Burnt Lime - A calcined material comprised chiefly of calcium oxide in natural association with lesser amounts of magnesium and is capable of slaking with water.
Calcium Carbonate Equivalent (CCE) - The acid neutralizing capacity of an agricultural liming material expressed as weight percentage of calcium carbonate.
Effective calcium Carbonate Equivalent (Effective calcium carbonate equivalent) - The percent of calcium carbonate equivalent (CCE) multiplied by the “fineness factor.”
Fineness - The percentage by weight of the material which will pass U.S. standard sieves of specified sizes.
Fineness Factor - The degree of fineness of the liming material used and shall be determined as prescribed under rules.
Hydrated Lime - A dry material made from burnt lime.
Industrial By-Products - Any industrial waste or by-product containing calcium or calcium and magnesium in
a form that will neutralize soil acidity and it may be designated by prefixing the
name of the industry or process used for its production.
Limestone - A material consisting essentially of calcium carbonate or a combination of calcium carbonate with magnesium carbonate capable of neutralizing soil acidity.
Marl - A granular or loosely consolidated earthy material composed largely of sea shell fragments and calcium carbonate.
§ 2-8-80.3. Distribution, Labeling and Sale of Liming Materials - Regulations.
- Agricultural liming materials sold, offered, or exposed for sale in the state shall
have affixed in a conspicuous manner on the outside of each package a plainly printed,
stamped or marked label, tag, or statement, or in the case of bulk sales, a delivery
slip or invoice, setting forth the following information:
-
- The name and principal office address of the manufacturer or distributor;
- The brand or trade name of the material;
- The identification of the product as to the type of the agricultural liming material;
- The net weight of the agricultural liming material; and
- The minimum percentage of Effective calcium Carbonate Equivalent (Effective calcium carbonate equivalent) guaranteed.
-
- No information or statement shall appear on any package, label, delivery slip, or advertising that is false or misleading to the purchaser as to the quality, analysis, type, or composition of the agricultural liming material.
- In the case of any adulterated material subsequent to packaging, labeling, or loading and before delivery to the consumer, a plainly marked notice shall be affixed by the vendor to the package or delivery slip to identify the kind and degree of adulteration.
- At every site from which agricultural liming materials are delivered in bulk and at every place where consumer orders for bulk deliveries are placed, there shall be conspicuously posted a copy of the statement required by this section for each brand of material.
- Each separately identified product or each effective calcium carbonate equivalent shall be registered before being distributed in this state. The application for registration shall be submitted to the Board on forms furnished. Upon approval, a copy of the registration shall be furnished to the applicant. The registration shall contain the labeling information required in subsection A of this section. Registrations shall be permanent unless canceled by the registrant or by the Board.
- A distributor shall not be required to register any brand of agricultural liming material that is already registered pursuant to the Oklahoma Agricultural Liming Materials Act by another person, providing the label does not differ in any respect.
§ 2-8-80.4. Information required by § 8-80.3 of this title to be affixed to containers.
- Any agricultural liming material offered for sale, sold, or distributed in this state
in bags, barrels, or other containers shall have placed on or affixed to the container
in written or printed form the information required by subsection A of Section 8-80.3
of this title, either:
-
- On tags affixed to the end of the package between the ears or on the sewn end or both between the ears and on the sewn end; or
- Directly on the package in a manner as determined by the Board.
-
- If distributed in bulk, a written or printed statement of the weight, as well as the information required by paragraphs 1, 2, 3 and 5 of subsection A of Section 8-80.3 of this title, shall accompany delivery and be supplied to the purchaser.
§ 2-8-80.5. Compliance With Act — Toxic Materials Prohibited — Administrative Penalty.
- No agricultural liming material shall be sold or offered for sale in this state unless it complies with provisions of the Oklahoma Agricultural Liming Materials Act or rules promulgated thereto.
- No agricultural liming material shall be sold or offered for sale in this state that contains toxic materials in quantities injurious to plants or animals.
- If an analysis shows that a commercial agricultural liming material falls below the guaranteed analysis, the State Board of Agriculture may require the payment of an administrative penalty to the consumer in the amount of the current value of the deficiency. All administrative penalties assessed pursuant to this section shall be paid to the consumer represented by the sample analyzed within thirty (30) days after the date of notice from the Board to the guarantor, with receipts taken and promptly forwarded to the Board. If the consumers cannot be found, the amount of the penalty shall be forwarded to the Board and be deposited in the State Department of Agriculture Revolving Fund.
§ 2-8-80.6. Vendor’s License for Spreading — Application — Fee.
- It shall be unlawful for any person to engage in the spreading of liming materials on properties belonging to others unless the person has a current vendor’s license issued by the State Board of Agriculture.
- Application for a license shall be in the form prescribed by the Board and shall state the name and address of the applicant and the number of spreader trucks or similar vehicles to be used by the applicant. The application shall be accompanied by an annual license fee of Twenty-five Dollars ($25.00). Each license shall expire December 31 of each year.
§ 2-8-80.7. Inspection Fees — Reports.
- For the purpose of helping to defray the expenses of inspection, administering, and carrying out the provisions of the Oklahoma Agricultural Liming Materials Act, an inspection fee of ten cents ($0.10) per ton shall be paid to the State Board of Agriculture on all agricultural liming material sold or distributed for use within this state.
- All agricultural liming material fees collected shall be deposited in the State Department of Agriculture Revolving Fund.
- Manufacturers, importers, and other guarantors distributing agricultural liming materials in the state shall file with the Board not later than the last day of January and July of each year, a semiannual report on forms furnished by the Board setting forth the number of net tons of agricultural liming material distributed in this state during the preceding six (6) calendar months. This report shall be accompanied by payment of the inspection fee. If no lime was sold or distributed in this state for the semiannual period, manufacturers shall submit a statement reflecting that information and shall remit a minimum fee of Five Dollars ($5.00). The Board shall have authority to audit records of each person to determine the accuracy of these reports.
- Any agricultural liming material on which the inspection fee has not been paid shall be subject to a stop-sale, removal order, or seizure.
- The Board may publish and distribute semiannually or annually to each person, distributor, registrant, licensee, and other interested persons a report showing the tons of agricultural liming material sold in Oklahoma. This report shall in no way divulge the operation of any registrant, distributor, or licensee.
Rules
35:30-31-1. Lime terminology
- Gypsum (CASO4) shall not be considered as an agricultural liming material.
- “Fineness” of a product shall be determined by passing a sample through a number eight (8) and number sixty (60) U.S. Standard Sieve, and calculating the percentage of weight of the material which passes through each sieve. The minimum “fineness” for any agricultural liming material distributed for use in Oklahoma shall be that 98% must pass through a four (4) mesh, 90% must pass through an eight (8) mesh and 30% through a sixty (60) mesh sieve.
- The “fineness factor” of a product shall be calculated as one-half (1/2) the percent passing through a number eight (8) sieve plus one-half (1/2) the percent passing through a number sixty (60) sieve equals “fineness factor”.
35:30-31-2. Lime vendor requirements
Lime vendors shall be responsible:
- To purchase, haul, and spread only limestone or other liming materials from manufacturers or producers who are registered in Oklahoma and reporting the inspection fee.
- To make sure all limestone or liming material is properly labeled when purchased from the manufacturer or producer; also that the product is properly labeled when delivered to the consumer.