Process and Facility Sanitation
- Jump To:
- Introduction
- Exceptions
- Housekeeping
- Processing and finished product controls
- Pest control
- Waste disposal
- Warehousing and storage
- Building exterior
- Floors and drains
- Walls and ceilings
- Ventilation
- Materials of construction for equipment, surface treatments and coatings
- Process design
- Equipment for product contamination
- Truck and railcar sanitation
- Employee service facilities
- Self inspections
- Conclusion
- References
Introduction
A general understanding of process and facility sanitation is important for the successful manufacturing of food products. When a new facility or process is designed, sanitation standards can be built-in. Good sanitation practices will improve product quality, minimize maintenance efforts, please inspectors and delight clients.
The Food and Drug Administration (FDA) enforces the Food, Drug and Cosmetic Act (along with some others) which covers all food commodities (except meat and poultry) in interstate commerce from harvest to distribution. Meat and poultry products are covered by the U.S. Department of Agriculture (USDA). The USDA has three areas of exclusive jurisdiction based on the following:
- Federal Meat Inspection Act
- Poultry Products Inspection Act
- Egg Products Inspection Act
These three acts are administered by the Food Safety and Inspection Service (FSIS) and give the USDA complete authority over all operations in the processing plants. These acts extend the USDA jurisdiction to the intrastate level. If the state inspection service cannot properly enforce the regulations, then the USDA will step in. If the USDA is not able to provide inspection services in this case, then the plant will not be allowed to operate.
Processed products containing meat in small amounts are not subject to the Meat Inspection Act if they meet the following three conditions:
- The product contains less than 3 percent meat.
- The meat must be comminuted so it cannot be distinguished as to identity, i.e. beef, pork, lamb, etc.
- The product name cannot include the name of the meat ingredient. However, the name of the meat must be included in the ingredient statement.
Exceptions
Poultry containing products must have less than 2 percent poultry (exception to #1), and “Pork and Beans,” (exception to #3). Exempted products come under FDA jurisdiction. Plants that process products that are under both FDA and USDA jurisdiction are inspected by both agencies.
Good Manufacturing Practices (GMPs) were developed to establish criteria in determining compliance with section 402 (a) (4) of the Food Drug and Cosmetic Act, particularly in terms of “sanitary” and “unsanitary” practices. GMPs have been revised over the years and the latest version is known as the current GMPs or cGMPs. The cGMPs for manufacturing, packing or holding human food can be found in the Code of Federal Regulations, Title 21, Part 110.
Housekeeping
The following points are key for proper housekeeping:
- Neat grounds surrounding the facility, free from rodent harborages, insect-breeding materials, debris, dust, weeds and odor producing conditions.
- Floors, aisles, ceilings, structural beams, piping, light fixtures and other overhead areas should be cleaned regularly.
- Restrooms, toilets, urinals and hand washing stations must be clean and functioning properly.
- Lockers and personal property storage areas should be clean and orderly.
- Drinking fountains and coolers must be clean.
Processing and finished product controls
- Food handling and processing equipment must be maintained in a clean condition, free of encrusted food residue, slime, mold and other contaminants.
- Open vessels must be protected from overhead contamination (figure 1).

Figure 1. Vessel with simple, hinged cover.
- All overhead catwalks and platforms (figure 2) must be designed and constructed to prevent accidental contamination of exposed foods or food contact surfaces.

Figure 2. Overhead catwalks must not allow particles to fall through into processing areas. Also, adequate kick guards should be included to prevent particles from being kicked into adjacent processing areas.
- Product and ingredient pipelines should be free of dead ends, pockets or any condition that leads to bacterial buildup (figure 3). Pipelines should be sloped to allow self-emptying.
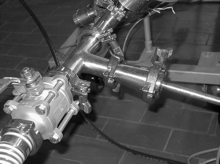
Figure 3. Dead leg in pipeline as shown at sensor location.
- Product and ingredient conveyors must be cleanable and self-emptying. They should have smooth, accessible surfaces, with open conveyors that are covered to prevent contamination.
- Water used as a food ingredient or for cleanup must be potable. The minimum requirement for water used as a food ingredient, personal purposes, and final washing of processed foods and cleaning of equipment is the Safe Drinking Water Act (Public Law 93-523) promulgated by the Environmental Protection Agency (EPA).
- Back flow and cross contamination must be prevented in all water connections. Never connect water lines directly to other process lines (figure 4). Water-line connections to tanks and vessels should have the required air gap (BOCA, 2000) to prevent back flow.

Figure 4. Direct water pipeline connections are prohibited! The laboratory retort shown in this photo is connected directly to the building’s water supply line (marked “A”). A check valve (“B”) and ball valve (“C”) are included in the plumbing, but the installation falls short of BOCA code, which requires an air gap or back-flow preventer.
- All machinery must be constructed to eliminate contamination of product. Grease and lubricants are important considerations.
Pest control
The four “D’s” for processors should be implemented:
- Do not feed pests
- Do not shelter pests
- Deny entry to pests
- Destroy pests
Keep plant grounds clean and free of uncut grass and weeds. Unused equipment, boxes, crates and other materials should be stored neatly to eliminate harborages. Plant entries should be fitted with tight doors, screens or air curtains (figure 5). Rodent bait box traps should be used around the exterior and inside of the plant (figure 6). Control measures must be implemented to keep processing and storage areas free of insects, rodents, birds, and other animal pests. Pesticides must be used with close supervision to prevent food contamination. A strip of pebbled rock (18 to 24″ wide and 4″ deep, of ¼” in diameter rock) around the entire facility is recommended for rodent control (figure 7). Concrete foundations should extend at least 2 feet below the ground and base of the foundation should extend outward in the form of an “L” at least one foot. The “L” extension should be four inches thick. Doorways and docks must be sealed with appropriate flashing and rodent brush. The physical abilities of rodents must be considered when rodent proofing a building. Rodents are capable of the following:
- Squeezing through small openings around pipes and cracks. All openings should be completely closed off. Rats can gain entrance to a building through an opening larger than ½ inch square and mice through an opening larger than ¼ inch square.
- Leaping up to 36 inches vertically and jumping up to 48 inches horizontally (rats).
- Climbing vertical walls, pipes, cables, trees, vines, etc.
- Falling (jumping) from substantial heights without injury: 8 feet for mice and as high as 50 feet for rats.
- Gnawing through substances as hard as cinder blocks and aluminum.

Figure 5. Air curtain installed above a roll-up dock door.

Figure 6. Rodent bait traps.

Figure 7. Pebble rock strip around production facility.
Waste disposal
Rubbish, offal and processing waste must be conveyed, stored and disposed of in a manner to prevent insect and rodent breeding or harborage, foul odors or food contamination. Marked and dedicated containers should be used for waste handling. Corridors and pathways used for waste handling and transportation should not be used for product or ingredient movement.
Warehousing and storage
All warehouse areas should be kept clean and orderly. At least 18″ should be maintained between walls and stored items as a “buffer zone” for cleaning and inspection. The floors in the buffer zone can be painted a light color to aid in detection of pests. Adequate aisle space must be provided for equipment movement. Stock should be routinely rotated and damaged stock should be clearly identified and separated. An incoming materials inspection and storage area is required. Incoming materials are checked for identity, shipping integrity, evidence of contamination, proper labeling, and conformity to standards or specifications. Often shipments must be fumigated before acceptance, requiring on-truck fumigation or a dedicated fumigation chamber. Foods returned from retail outlets must be clearly identified and stored in a designated area.
Building exterior
The facility does not need to be an award winner, but an attractive plant is more readily accepted by the community and provides a better work environment. Landscaping should be simple and neat, avoiding anything that could be a harborage for pests or promote dust. Outdoor lighting should be designed to provide security and convenience to employees. Unsightly areas can be fenced.
Floors and drains
Slope of floors should be at least ¼” per foot. Many consider that dairy brick (figure 8) is the ideal floor surfacing material, but the cost is prohibitive. Epoxy is affordable and provides a durable covering. The obsolete USDA standard for poultry drains specified drain locations not more than 10 feet from the highest point of the floor. A 12″ by 12″ drain with a 4″ to 5″ diameter pipe is usually adequate (figure 8). Drain vent openings to the outside must have rodent screens. Deep seal traps P, U and S type (not ball) are acceptable. Metal screw plugs should be provided for drains in areas where water in the plumbing trap may evaporate, allowing sewer gases to escape.

Figure 8. Floor drain and dairy brick flooring surface.
Walls and ceilings
Wall surface materials should be impervious to water in wash-down areas. Unpainted surfaces are best. Windowsills should be at a 45-degree angle or more to prevent buildup of dust and soil. Caulked or otherwise sealed seams tend to grow mold over time, but this situation is often unavoidable. Any insulating material is suitable, so long as it is completely sealed from water and insects. Closed-cell type insulation is waterproof and may provide a superior barrier to insects. The presence of insulation of any type or quantity in food products is considered a contaminant.
Drop-down utilities (electric, steam, gas, compressed air, etc.) are simpler to clean and do not clutter the plant floor (figure 9). An overhead machinery loft area can improve plant appearance, reduce cleaning costs, improve lighting, minimize overhead contamination concerns and improve access for maintenance. False ceilings are discouraged because of insect harborage, but may be acceptable under certain conditions, such as the inclusion of maintenance catwalks, sealed seams and automatic pesticide applicators.

Figure 9. Drop-down utilities.
Ventilation
Two key design aspects for ventilation systems are (1) positive air pressure in food processing areas, and (2) air pressure gradients designed to prevent cross-contamination between finished goods and raw product areas. Positive pressure prevents contaminated, outside air from entering into the building. Air filtration requirements depend upon the application and area of the plant. Air pressure gradients, in general, should be higher at the location of finished product and lower at the point of raw product processing. Air pressure gradients maintained in this manner will prevent airborne particles generated from the raw product from contacting the finished product.
Ventilator openings should be protected with gratings and screens. Condensation on facility ceilings must be eliminated or captured and controlled. Dripping condensate can enter product as a potential contaminant. Cleanable ventilation ductwork and condensate traps (figure 10) and drains in ductwork are often necessary. This can be especially true in freezers and refrigerators where condensation can occur on chilling units. In the case of freezers, the condensate drain line must be heated (to prevent freezing) and insulated.

Figure 10. Condensate trap and catch pan mounted on interior edge of ventilation hood.
Materials of construction for equipment, surface treatments and coatings
Corrosive activity of the product and cleaning compounds must be considered when selecting materials of construction. If the process is wet or requires wet cleaning, then stainless steel or another non-corroding material is preferred. Painted surfaces are almost never satisfactory under wet conditions. For dry process areas, epoxy-painted, mild steel is adequate. Orientation of structural shapes should be specified to eliminate ledges and flat surfaces where dust, dirt and grime can accumulate. For example, angle iron is acceptable in the horizontal plane when the vertical leg points down. It can also be used in the horizontal plane with the heel pointing up. Channels should be used with the web in the vertical plane or flanges pointing down if it is in the horizontal plane. H or I beams should not be used with the web in the horizontal plane. Open ends of piping must be completely sealed, preferably welded. Flat plate steel in horizontal orientation should be avoided. Angle and channel legs for equipment support are difficult to clean where they contact the floor (because of the included angle). Figure 11 shows proper installation (a) and poor installation (b) of electrical switchgear.

Figure 11. Good, wall-mounted recessed electrical installation (top) and less desirable electrical installation (bottom) that includes more horizontal surface area for soil accumulation.
Process design
Key elements of good process and packaging flow layout are:
- Ample space for cleaning and maintenance.
- Smooth orderly flow of materials through each manufacturing phase.
- Use of gravity where possible.
- The shortest distance between two points.
Equipment should be placed with a minimum of 36″ of clear space for sanitation. A maintenance envelope for each piece of equipment should also be considered. Overhead clearance should be a minimum of 18″ to allow for cleaning. Elevation above the floor should be at least 6 inches or the equipment should be completely sealed to the floor. Elevation of equipment is preferred, since seals will eventually leak and contaminants will be trapped between the equipment and floor. Larger (wide) pieces of equipment might require more floor clearance for cleaning purposes. A well-composed equipment arrangement may place identical pieces of equipment (such as motors, tanks, spouts and pipes) together. “Gray-side” maintenance areas for piping, ductwork and equipment are desirable to separate potential sources of contamination from the product. The so-called gray side of the process equipment is separated from the main process area by a barrier wall or ceiling. Examples are hydraulic drives located on the second floor with fluid-power piped down to the “clean” process on the first floor or a complex filling machine that is built into a wall with the drive and mechanical components accessed from the gray side and the filling components on the clean side.
Equipment for product contamination
Reduction
Foreign material control involves both chemical and physical contamination of the food product. Control can take place in both operations and design. Reducing chemical contamination involves using only approved chemical substances near food products and controlling the application and use of chemicals.
The primary focus of process design is the development and operation of a process that does not generate or add contaminants to the product. Assuming that some contaminants will be included or generated, there are six important classes of equipment that can be used to detect and remove them: magnets, metal detectors, sifters and scalpers, aspirators, color sorters and strainers. Magnets and metal detectors are normally used at four specific locations:
- Receiving (ingredients and raw materials).
- Before certain pieces of equipment as a protective measure to prevent the equipment from being damaged by contaminants.
- After equipment that may cause contamination.
- At the point of primary packaging.
A photograph of a metal detector is shown in figure 12. Scalpers and sifters (figure 13) are most frequently used for grading and classifying and cleaning raw commodities such as flour, grains, beans and vegetables. These devices are sometimes successfully used to remove insect contamination. Aspirators are used to remove infestation, weed seeds and other contaminants from a variety of grains and some finished products such as breakfast cereal. Color sorters identify contaminants based on their color difference when compared to a given standard. These units have been successfully used to remove stones from peas, and culls from sliced vegetables. Line strainers and pipeline magnets (figure 14) remove contaminants from liquids. Line strainers and pipeline magnets in a continuous process should be installed in a duplex arrangement. Duplex magnets will allow the cleaning and inspection of one unit while the other unit remains on line.

Figure 12. Conveyor-mounted metal detector and reject mechanism.

Figure 13. Sifter

Figure 14. Pipeline magnet.
Truck and railcar sanitation
Major sanitation problems can be caused by mixed loads of materials resulting in contamination. The following sanitation problems are recurring issues for trailers and railcars:
- Insect and rodent infestation and contamination.
- Foreign substances such as root, sand, debris, glass, oil and garbage.
- Microbiological and or chemical contaminants.
- Off odors, often undetected at time of loading.
The FDA is responsible for inspecting interstate delivery vehicles and carries out an ongoing “Carrier Sanitation” program. Many companies do not salvage goods that have been damaged or contaminated during transportation. Salvage activities often cost more than the goods are worth and the risks of creating a pest harborage are not justified. A salvage activity must be carefully evaluated before being established and will require a great deal of planning and dedicated management.
Employee service facilities
Standards exist in documents (see reference list) published by local, state and federal organizations. The applicable standards and codes should be carefully researched before any work is started.
The following general recommendations are given in no particular order:
- Changing rooms must be provided for each sex when employees change clothing routinely because the work involves dirt, heat, fumes, vapor or moisture.
- Locker rooms should be designed to eliminate storage of food and clutter. The facility should be arranged to keep street and work clothes separate. If damp clothing is routinely stored in lockers, the lockers should be heated from beneath. Other housekeeping problems associated with lockers can be partially remedied by constructing lockers flush with the floor or on a raised base to permit easier cleaning, installing lockers with sloped tops to prevent accumulation of dust and debris and ventilating lockers to reduce odors.
- Hand-washing facilities must be located in or near toilet rooms. Hand-washing facilities must also be accessible to food handlers near their job activity site. Heavy traffic periods of beginning work, breaks and lunch should be accommodated without unreasonable waiting periods. One washbasin is recommended per 10 employees up to a total of 100. After 100 the figure increases to one basin per 15 employees. Twenty-four inches of sink space with an individual faucet is equivalent to one basin. Hand-washing facilities should be plentiful and easy to access, or they will not be used, especially on peak-demand periods. Stations must be designed to provide ample water at the proper temperature on demand, with “hands-free” operation (figure 15). Suitable drying materials should be available; warm-air blowers are not recommended, since they require too much time.
- Foot wash stations may be required in hallways and entrances where cross-contamination is possible or where soiled shoes are a factor. Heavy soils may require the additional measure of manual or powered sole scrubbers.

Figure 15. Hands-free sink station.
Other employee service rooms that may be needed are:
• Training room
• Retiring room
• First aid
• Food service facility
• Lunch room
• Vending machine room or area
Self inspections
A self-inspection program is necessary for good preventative sanitation and to keep the management fully aware of problem areas. Customized inspection forms should be developed to fit the needs of the facility. Self-inspection is important for the following reasons.
- Customers do not want to receive or deal with infested, contaminated or adulterated merchandise. It makes good business sense to make your company clean and reputable.
- The food laws and regulations that FDA enforces apply to firms that receive or ship food in interstate commerce. The requirements place the prime legal responsibility for clean, safe, and quality foods, upon the food processor.
In terms of cost and manpower, self-inspection is the most reasonable means of helping to ensure a satisfactory food processing and storage operation and a “clean bill of health.” If self-inspection is carried out on a regular basis, potential problems can be corrected proactively.
A generic checklist has been provided to indicate a variety of items that should be periodically inspected. This checklist can serve as a guide to help maintain or improve facility sanitation; it should be modified to fit a particular facility.
Conclusion
Proper process and facility sanitation practices will contribute to improved product quality and wholesomeness and the “bottom line” of the company. The information presented herein provides a starting point. All sanitation practices must be individually developed to suit the particular facility and process of concern. Maintenance and development of sanitary conditions are an ongoing process, to be continually evaluated an improved.
References
BOCA 2000. International Building Code. Building Officials Code Administrators International. Country Club Hills, IL.
Food and Drug Administration. 1998. Code of Federal Regulations. Title 21, Chapter 1, Part 110. Current Good Manufacturing Practices in Manufacturing, Packing or Holding Human Food. U.S. Government Printing Office, Washington, D.C.
Occupational Safety and Health Administration (OSHA), U.S. Department of Labor. Regional Office: 512 Griffin Street, Room 602, Dallas, TX 74202, phone 241-767-4731, fax 214-767-4137. Website: http://www.osha.gov/
U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition, 1994 “Do Your own Establishment Inspection, A Guide to Self Inspection for the Smaller Food Processor and Warehouse.”
Oklahoma Department of Agriculture. P.O. Box 528804, Oklahoma City, OK 73152, 405-521-3864.
Website: http://www.state.ok.us/~okag/index.htm
Shapton, D.A. and N.F. Shapton (editors). 1998. Principles and practices for the safe processing of food. Woodhead Publishing Limited. Cambridge, England.
The University of Georgia College of Agricultural and Environmental Sciences , Cooperative Extension Service, “Cleaning, Sanitizing, and Pest Control in Food Processing, Storage and service Areas,” Prepared by George A. Schuler, Maxcy P. Nolan, A.E. Reynolds and W.C. Hurst, 1999.
United States Department of Agriculture Food Safety and Inspection Service, Washington, DC. Website: http://www.fsis.usda.gov/
Tim Bowser
FAPC Food Process Engineer
Jason Young
FAPC Quality Management Specialist
