Nutrition Facts Panel Changes: Combating an Old Problem with a New Look
Introduction
Since 1994, the Food and Drug Administration has been combating the growing obesity epidemic in the United States through the use of the Nutrition Facts label, the “Spot the Block” program, the formation of “FDA’s Obesity Working Group” and Michelle Obama’s “Let’s Move!” Campaign. Now, the iconic Nutrition Facts label, found on processed and packaged foods, is being reformatted by the FDA to reflect the eating habits of the current population and give consumers a greater understanding of nutrition. Michael R. Taylor, FDA’s deputy commissioner for foods and veterinary medicine, said, “By revamping the Nutrition Facts label, FDA wants to make it easier than ever for consumers to make better informed food choices that will support a healthy diet,”
New Nutrition Facts Label: What's different and why?
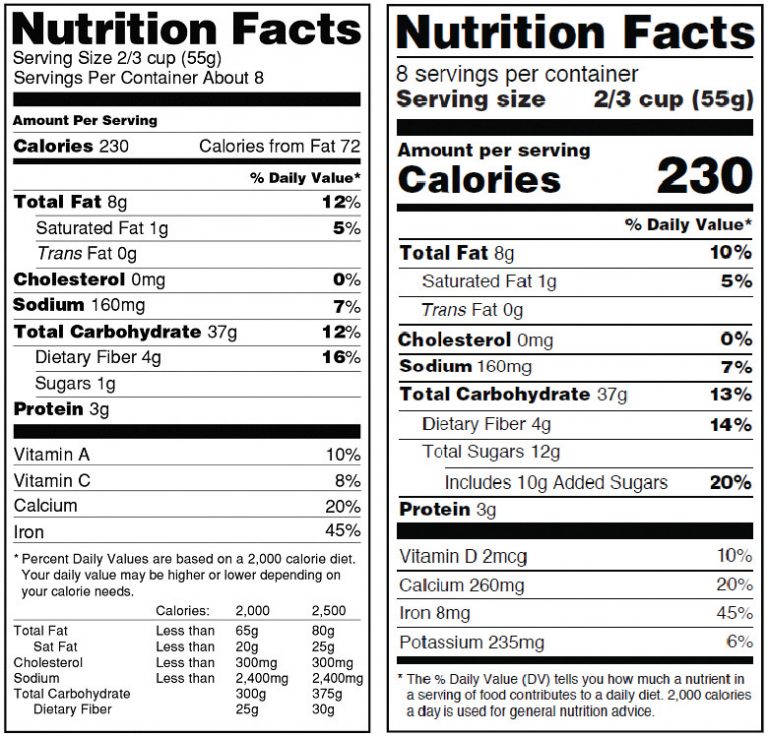
In an attempt to help consumers better understand the nutritional value of what they are buying and later eating, the FDA is proposing a few adjustments to the Nutrition Facts label. On March 3, 2014, the FDA proposed a new rule that would improve the nutrition label as a tool to help consumers make better, more informed food choices and maintain healthy lifestyles. As a result, these adjustments are meant to bring more attention to the information consumers generally seek (calories and serving size), avert their attention to other important nutrients and explain Percent Daily Value in more comprehensible terms. While the adjustments are distinct, the overall effect does not change the iconic label too much. Below is a list of the changes and short reasons why the changes have been made. For more in-depth information about all the changes and their reasonings visit the FDA website.
Table 1. Changes and Reasoning for Changes to Nutrition Facts label
| Proposed Change | Reasoning |
|---|---|
| Vitamin A and C will no longer be required. | Current data indicate that Vitamin A and C deficiencies in the general population are not common. These vitamins still would be allowed to be declared on labels on a voluntary basis. |
| Vitamin D and potassium will be added to the Nutrition Facts label. | Vitamin D is important for its role in bone health, and some population groups are not getting enough of it. Adequate potassium intake is beneficial in lowering blood pressure and intakes of this nutrient are low among some population groups. |
| The “calories and serving size per container” type size will be increased and bolded. | Many consumers use this information in assessing the nutritional value of the product before buying. The FDA hopes the changes will bring more emphasis to parts of the label that are important in addressing current public health concerns such as obesity, diabetes and cardiovascular disease. |
| The information on Percent Daily Value will be shifted to the left of the label. | In order to convey the importance of the Percent Daily Value, the shift to the left places the nutrient amounts of the food first and foremost. The intent is to help consumers learn that the Percent Daily Value tells you how much of certain nutrients you are getting from a particular food in the context of a total daily diet. |
| The actual amount of mandatory vitamins and minerals,and those volunteered will be declared. | Reason not specified. |
| Total Carbohydrate will be replaced by “Total Carbs." | Reason not specified. |
| Total Carbohydrate will be replaced by “Total Carbs." | The proposed rule would require declaration of “Added Sugars” as well, indented under “Sugars,” to help consumers understand how much sugar is naturally occurring and how much has been added to the product. This proposed change is based on expert recommendations –including those from the 2010 Dietary Guidelines for Americans, that Americans should reduce their intake of calories from added sugars. |
| Serving size information will be right-justified. | Reason not specified. |
| “Amount per Serving” will change to “Amount per ___” | The blank will be filled in with the serving size in common household measurements (e.g. Amount per ¼ cup). |
| Serving Size references will be updated. | New serving sizes will reflect how much food people actually consume today in order to give consumers more accurate information regarding the caloric content in the product. Rather than be diminished, by law, serving sizes must be based on how much food people actually consume and not on what they should eat. For some food manufactures, the serving sizes on their product may potentially increase or decrease as new studies have shown 17 percent of the reference amounts customarily consumed used to calculate serving sizes should be changed. |
| The footnote will be replaced with new information. | The new information will better explain the Percent Daily Value. |
To demonstrate the effects of the proposed changes, Figure 1 provides a side-by-side comparison of the current Nutrition Facts label and the proposed label.
Figure 1. Comparison of previous and proposed nutrition label formats.
For packages containing more than can be eaten in one sitting, there will be a dual column version of the Nutrition Facts label. The left column will have the calorie categories (e.g. total fat, cholesterol, vitamin D, etc.), the middle column contains the calorie content information for a single serving, and the right column will contain the calorie content information for the entire container or package. The FDA will require this format for packages that contain at least two times the serving size and less than or equal to four times the serving size. (See Figure 2.)
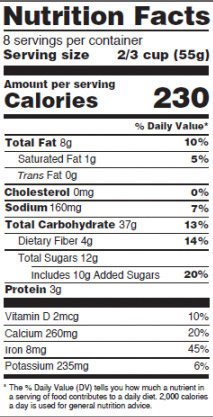
Figure 2. Proposed multi-serving Nutrition Facts label.
Should the proposed changes be accepted for all packaged food, including imports, manufacturers will need to comply with these new regulations within two years after the effective date. Foods exempt from the updated label are those regulated by the U.S. Department of Agriculture’s Food Safety and Inspection Service (i.e. certain meat, poultry and egg products). For more information visit the FDA website.
References
Food & Drug Administration. 2015. “Proposed Changes to the Nutrition Facts Label.”
Food & Drug Administration. 2015. “Proposed Changes to the Nutrition Facts Label.”
Food & Drug Administration. 2014. “FDA Proposes Updates to Nutrition Facts Label on Food Packages.”
Food & Drug Administration. 2014. “Food Serving Size Getting a Reality Check.”
Food & Drug Administration. 2014. “FDA News Release: FDA proposes updates to Nutrition Facts label on food packages.”
Meagan Osburn
FAPC Business & Marketing Intern
Chuck Willoughby
FAPC Business & Marketing Relations Manager
Rodney Holcomb
FAPC Agribusiness Economist