Anaerobic Digestion of Animal Manures: Inhibitory and Toxic Materials
Most organic substrates, added in small doses, increase biogas production in a manure digester. Larger doses of the same material may decrease, or inhibit, biogas production. In other words, too much of a good thing may be toxic to the digester. This Fact Sheet explains how to test for potentially inhibitory substances, identifies the most common materials that inhibit gas production, and gives advice on how to deal with a toxic element once it has been identified.
What is Toxicity?
“Toxic” is a relative term. To a biologist, “toxicity” implies a dose response. A small dose of a toxic material has a small response, large doses have large responses, and an even larger dose can be lethal. It is better to think of toxic materials as inhibitory. Above a certain concentration in a digester, the presence of an inhibitory material causes biogas production to decrease. Many inhibitory materials actually stimulate gas production in small doses. For instance, a small amount of ammonia provides nutritious nitrogen to a digester’s microorganisms. A large dose of ammonia disrupts methane production.
Anaerobic Toxicity Assay
Adding a co-digestion product to boost biogas production in manure digesters is becoming a common practice. Many farmers base their decision to use a particular co-digestion product on a positive Biochemical Methane Potential (BMP) test. This could be a costly error. The BMP test determines the potential for a co-digestion product to produce methane (CH4) under ideal conditions and low doses. (For more information on the BMP test, see OSU Fact Sheet BAE 1762, Anaerobic Digestion of Animal Manures: Methane Production Potential of Waste Materials.)
The primary method to determine whether a chemical is stimulatory, inhibitory, or lethal to digesters is to perform an Anaerobic Toxicity Assay, sometimes called an ATA test. The ATA is similar to a BMP test. Seed organisms (sometimes called inoculum) are introduced to an airtight container, and the amount of biogas or methane (CH4) released during incubation is recorded. The seed organisms are fed a fixed amount of an easily digested solution such as glucose, acetate, or yeast extract. Increasing amounts of a potentially toxic ingredient are added to the test bottles. If the ingredient is toxic to microorganisms, gas production decreases as the amount of ingredient is increased. If the ingredient is not toxic, the amount of biogas released remains the same or increases as more of it is added.
Incubation temperature of an ATA test is usually 35 C –the optimum temperature for
mesophyllic organisms. Other temperatures can be used depending on operating temperature
of the digester in question. Due to the high variability expected when dealing with
a living system, the control and each sample is prepared in triplicate. Seed organisms
are cultured in a dedicated reactor, and the same seed is used for each sample and
control. Gas production is monitored by either collecting gas from, or measuring pressure
increases in, the test bottles. The International Organization for Standardization
procedures for ATA testing (ISO 13641-1, 2003, and ISO 13641-2, 2003) recommend monitoring
total biogas production; however, since the first indication of toxic inhibition is
a shift in biogas composition from CH4 to Carbon dioxide (CO2), it is more accurate
to gauge inhibition by measuring CH4 produced.
Results of ATA tests are recorded as percent inhibition, (I):
I = (1 – Pt/Pc) X 100 (1)
Where Pc is the average volume or pressure of gas produced in the control bottles
(no toxic agents added), and Pt is the average volume or pressure of test bottles.
Positive numbers indicate that the material is inhibitory at the concentration added.
Negative or zero I values indicate no inhibition. Gas production is measured for several
concentrations of the toxic material in order to determine its dose response.
Statistical tests are often performed on the dose response curves to calculate the
IC10 and IC50 points. The abbreviation IC stands for maximum inhibitory concentration. The IC is
related to another measure called the maximum effective concentration or EC, which
is the measure of a drug’s potency. For antibiotics, IC and EC are essentially the
same measure, since effect of an antibiotic is to kill or inhibit the growth of microorganisms.
The IC10 point as relates to an ATA is the concentration of the toxic material that causes
a 10 percent drop in gas production. Likewise, the IC50 is the concentration that leads to a 50 percent decrease in gas production.
ATA on a Single, Potentially Toxic Ingredient
When an ATA is performed on a single element, such as an antibiotic or sanitizer, the potentially toxic material is generally increased in a geometric addition. In other words, the amount of toxic material is doubled with each addition. Table 1 gives an experimental set up for an ATA run on the antibiotic, Imipenem. (Imipenem is a broad spectrum intravenous antibiotic. It is used in combination with cilistatin, and marketed in the USA under the trade names Primaxin, Tienam, and Zienam.) In this experiment, the inoculum was dried, anaerobically digested, sewage sludge. The standard feedstock was yeast extract supplemented with minerals. Distilled water was added to bring the volume in each test bottle to 150 ml.
Table 1. Anaerobic Toxicity Assay Experimental Design for a Single Toxic Element (from Gartiser et al, 2007).
| Dosage | Control | 6.25 mg/l | 12.5 mg/l | 25 mg/l | 50 mg/l | 100 mg/l |
|---|---|---|---|---|---|---|
| Inoculum (mg) | 150 | 150 | 150 | 150 | 150 | 150 |
| Standard Feedstock (mg) | 375 | 375 | 375 | 375 | 375 | 375 |
| Imipenem (mg) | 0 | 0.9 | 1.8 | 3.75 | 7.5 | 15 |
| Total Liquid Volume (ml) | 150 | 150 | 150 | 150 | 150 | 150 |
| Number of Bottles | 5 | 3 | 3 | 3 | 3 | 3 |
Gas production over seven days for each dose of Imipenem is shown in Figure 1. Percent inhibition, I, for each dose was calculated using cumulative gas production at day 7, and the dose response curve is graphed in Figure 2. Figure 2 shows that Imipenem inhibits biogas production at all concentrations tested. Each dose tested had a positive I value, and while I did not increase with each doubling of Imipenem, the trend is greater inhibition at higher doses. The experimenters used a computer program to determine the EC10 to be 0.7 mg/l (95 percent confidence interval for EC10 was below detection limit to 3.5 mg/l). Likewise, the EC50 was calculated as 24.2 mg/l (95 percent confidence: 8.5 to 64.5 mg/l).
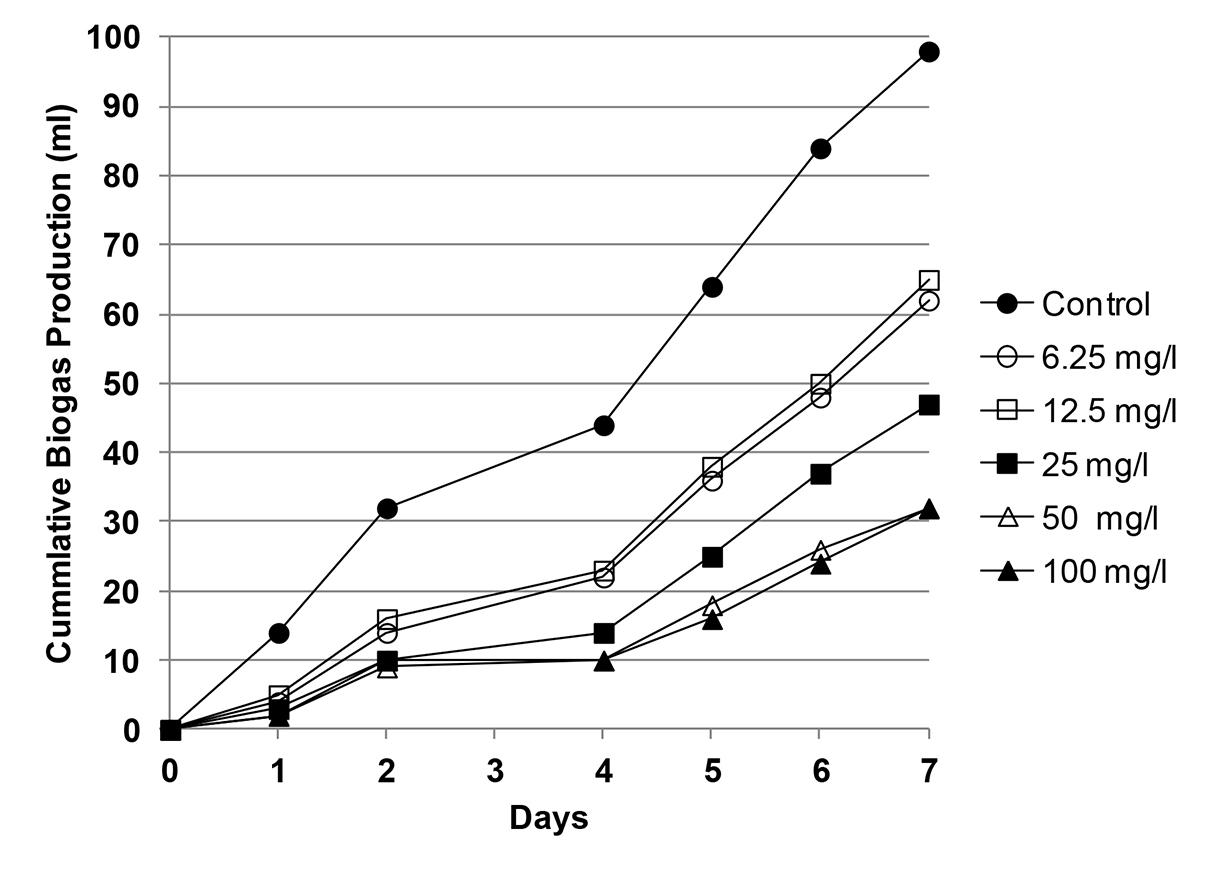
Figure 1. Biogas Production over Seven Days at Various Doses of the Antibiotic Imipenem (from Gartiser et al., 2007).
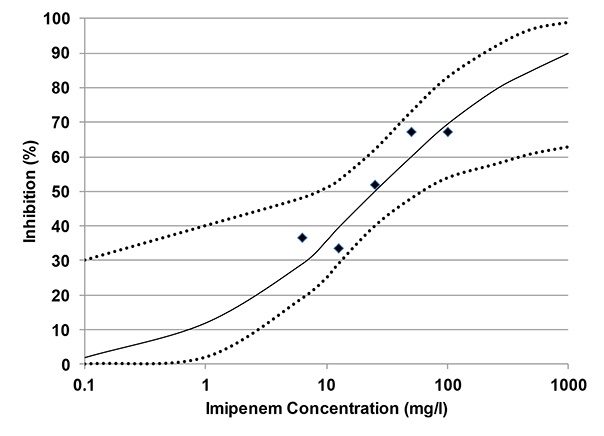
Figure 2. Measured Inhibition from the Antibiotic Imipenem, and Calculated Inhibition Function with 95 percent Confidence Interval (from Gartiser, et al, 2007).
ATA on Mixtures of Materials
When an ATA is run on a mixture of potentially toxic materials, testing is done on a percent inclusion basis. In other words, the dosage tested is the percentage of liquid test material added, divided by the entire test volume. A typical ATA test setup to test toxicity of co-digestion products is given in Table 2. The volume of inoculum (mixed liquor removed from a dedicated reactor) and standard feedstock (glucose solution) remain constant across the test (50 ml inoculum, and 2 ml standard feedstock). Fifty ml of test solution, which is a mixture of co-digestion product and deionized water, is added to each bottle containing inoculum and standard feedstock, bringing total volume to 102 ml. The volume of co-digestion product is increased in the test solution from 0 (control) to 50 ml (49 percent inclusion). Volume of distilled water decreased in proportion to substrate added. Percent inclusion is calculated by dividing the volume of the co-digestion product by the total volume of the test (1 ml co-digestion product/102 ml total 100 ≈ 0.01 or 1 percent. 50 ml co-digestion product/102 ml total X 100 ≈ 49 percent).
Table 2. Anaerobic Toxicity Assay Experiment Design for Co-digestion Products (from Moody et al, 2011).
| % Inclusion | Control | 1 | 10 | 15 | 25 | 34 | 44 | 49 |
|---|---|---|---|---|---|---|---|---|
| Inoculum (ml) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Standard Feedstock (ml) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Co-Digestion Product (ml) | 0 | 1 | 10 | 15 | 25 | 35 | 45 | 50 |
| Deionized Water (ml) | 50 | 49 | 40 | 35 | 25 | 15 | 5 | 0 |
| Total Volume (ml) | 102 | 102 | 102 | 102 | 102 | 102 | 102 | 102 |
| Number of Bottles | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
Results of ATA tests run on two co-digestion products are given in Figure 3. Both substrates were food processing byproducts. One of the byproducts is inhibitory at all inclusion rates; whereas, the second byproduct appears to have no toxicity. In Figure 3, negative values of I mean more CH4 was produced with the addition of non-toxic material compared to the control. More substrate means more food was available for the microorganisms in the inoculum to make CH4. On the other hand, adding the toxic co-product decreased CH4 production, thus increasing percentage inhibition.
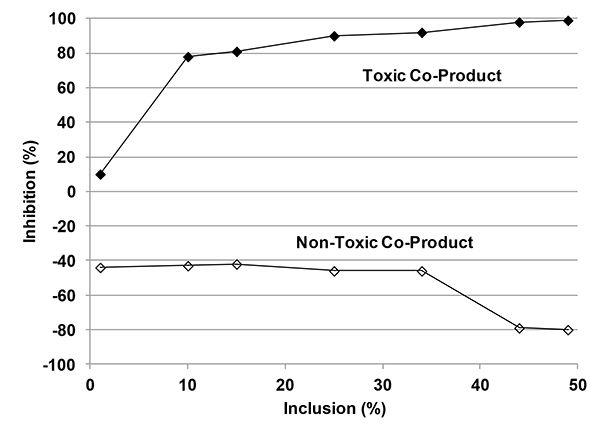
Figure 3. Inhibition Measured in an Anaerobic Toxicity Assay for a Toxic and a Non-toxic Co-digestion Product. (From Moody, et al., 2011).
Common Inhibitory Materials
An ATA run on a potentially toxic material indicates whether the material stimulates or inhibits biogas production. It also provides a first cut at determining the maximum loading rate of the material. The ATA test does not indicate the reason why the material is toxic. The following are the most common inhibitors of digester performance.
Antibiotics
Antibiotics given therapeutically or fed sub-therapeutically have the potential to alter the microbial communities digesting manure excreted by treated animals. Results of ATA tests using antibiotics show definite inhibition of biogas production at low doses (Table 3). Sodium Monensin did not have a measured dose response, but decreased CH4 production 77 percent at doses as low as 25 mg/l. Rumensin, the sub-therapeutic form of the drug, had an IC50 of 0.1 percent or 1,000 mg/l (assuming an aqueous solution, a 1 percent inclusion rate is equivalent to 10,000 mg/l.).
Table 3. Half Maximum Inhibitory Concentrations (IC50) of Various Chemicals Based on Mesophyllic Anaerobic Toxicity Assays Reported in the Scientific Literature.
| IC50 | |||
|---|---|---|---|
| Common Name | Composition | % Inclusion | mg/l |
| Antibiotics | |||
| Amoxicillin | 2,7201 | ||
| Ceplasporin | Cefriaxone disodium salt | 3071 | |
| Lincomycin | Clindamycin | 1971 | |
| Chlortetracycline | Chlorotetracycline HCL | 43.41, 402 | |
| Chloramphenicol | 262 | ||
| Monensin | Sodium Monensin | 77% Inhibition between 25 and 100 mg/l without dose response1 | |
| Rumensin™ | 80 ppm Sodium Monensin, feed additive for cattle | 0.13 | |
| Penicillin G | Benzylpenicillin Sodium Salt | 4,7951 | |
| Tetracycline | 37.31 | ||
| Tylosin | >2603 | ||
| Sanitizers and Cleaners | |||
| Genron IV™ | Quaternary Ammonium chloride and Ethanol | 0.13 | |
| Della Super™ | Chlorine Alkaline Clean in Place Sanitizer | 0.73 | |
| Tri pfan™ | Chlorine Alkaline Clean in Place Sanitizer | 0.83 | |
| Zinicin™ | Chlorine Alkaline Clean in Place Sanitizer | 1.13 | |
| 1313-SD™ | Chlorine Alkaline Clean in Place Sanitizer | 1.83 | |
| Sheen Ezey™ | Chlorine Alkaline Clean in Place Sanitizer | 1.13 | |
| Mandate™ | Phosphoric, Octanoic, Citric, and Decanoic acid | 1.83 | |
| Disinfectants | |||
| Hoof Dip | 30 g/l, 98% pure Copper sulfate | 4.03 | |
| Masticide™ | Phenol | 1.03 | 2,1004 |
| Artec™ | Heptanoic Acid | 4.03 | |
| Della-Soft™ | Iodine | 5.83 |
1 Gartiser, et al, 2007.
2 Sanz, et al, 1996.
3 Zitomer, et al, 2007.
4 Blum and Speece, 2012.
Antibiotics fed to animals are partially metabolized as they pass through the digestive tract, but the altered forms of the chemicals, or metabolites, may also act as inhibitory agents. Varel and Hashimoto (1982) found that digesters fed manure from Rumensin treated cattle showed complete inhibition after three weeks; however, if the digesters were continuously fed the Rumensin altered manure, they made a full recovery of gas production in six months. Most antibiotics are bactericidal agents. That is, they kill microbial populations. However, if the entire microbial population is not wiped out, some of the offspring of the survivors may develop resistance to the antibiotic. This explains why gas production in digesters receiving Rumensin recovered after six months. Whether it was the microorganisms in the digester or the microflora of the cattle’s intestines that became resistant to Monensin is not clear. Although this is good news for digester operators, it may indicate a source of antibiotic resistant microorganisms in the environment.
Cleaners, Sanitizers, and Disinfectants
Inhibitory response to chemical antimicrobial agents measured by ATA tests is similar to that of antibiotics (Table 3). Disinfectants, sanitizers, and cleaners are generally bacteriostatic agents. They do not kill organisms, but inhibit microbial growth at sufficiently large doses. Populations usually recover if the inhibitory dose of the bacteriostatic agent is removed. If used properly on the farm, the doses of cleaning and sanitizing chemicals reaching a digester should be much lower than the IC50 concentrations shown in Table 3. For instance, the C50 of the Copper sulfate (CuSO4) hoof dip is 40,000 mg/l. Consider the path CuSO4 takes to reach a digester. Dairy cattle walk through a trough of hoof dip as they enter a milking room, loafing area, or barn. A small amount of CuSO4 is transferred to the floor as they walk. This small volume is diluted with much larger volumes of wash down and flush water before it enters a digester.
Ammoniacal Nitrogen
Dissolved ammonia gas (NH3) and its ionized form, ammonium ion (NH4+), are byproducts of protein digestion and reduction of urea. Concentrations at which ammoniacal nitrogen (NH4+NH3-N) are beneficial, inhibitory, or toxic to anaerobic digestion are given in Table 4. The toxicity of ammoniacal nitrogen is highly dependent on pH. Ammonia gas, which is the predominant form at higher pH, is more toxic than NH4+. Ammoniacal nitrogen is usually not a problem in agricultural digesters except for reactors treating highly nitrogenous materials such as poultry manure or food byproducts.
Table 4. Effect of Ammoniacal Nitrogen and Sulfide Concentrations on Anaerobic Treatment (from McCarty, 1964).
| Effect on Anaerobic Treatment | NH4+NH3-N mg/l |
S- mg/l |
|---|---|---|
| Beneficial | 50 -- 200 | < 50 |
| No Adverse Effect | 200 – 1,000 | 50 - 100 |
| Inhibitory at higher pH values | 1,500 – 3,000 | 100 - 200 |
| Lethal | > 3,000 | > 200 |
Sulfate and Sulfide
Sulfate ions (SO4–) are present in urine. They are also created during the aerobic breakdown of proteins containing the amino acids cysteine and methionine. Sulfate is not an inhibitory substance to digesters per se. Some methanogens produce CH4 from CO2 and Hydrogen (H2). Sulfate reducing bacteria also use H2 to make Hydrogen sulfide (H2S) from SO4–. Since sulfate reducers grow faster than methanogens, they usually win the battle for H2, and CH4 production suffers as a consequence. This is generally not a problem with manure digesters, because there are plenty of other pathways for methanogens to produce methane with manure as a feed source. (See OSU Fact Sheet BAE-1747, Anaerobic Digestion of Animal Manures: Understanding the Basic Processes.)
The end product of sulfate reduction, Sulfide ion (S–), is quite toxic to anaerobic digestion (Table 4). Like ammonia, the toxicity of
S– is pH dependent. At lower pH, S– exists in its gaseous form, H2S. While H2S is less toxic to digesters due to its low solubility in water, it is highly toxic
to humans. It is also corrosive and will quickly destroy electronic equipment. The
toxicity of sulfide is reduced through precipitation of insoluble metal sulfides,
which also happens to be a good method of reducing toxic levels of Nickel, Zinc, and
Iron.
Heavy Metals
Heavy metals are generally not a concern in manure digesters. Exceptions are the metals Nickel and Zinc, which can sometimes accumulate to inhibitory levels. Other potentially toxic metals are Arsenic and Chromium, which are used rarely in special diets. Check feed labels for the presence of metals that could potentially enter a digester. Other sources of heavy metals are treated lumbers and lawn fungicides. It is a good idea to check the source of sawdust or grass clippings before adding them to a digester.
Soluble Salts
The higher the salt content of a liquid, the harder microorganisms have to work to transport water in and out of their cells. The toxic action of salts is predominately determined by the cation (the positive ion in solution with a negative anion). Table 5 lists the stimulatory and inhibitory ranges of base cations (Sodium, Potassium, Calcium, and Magnesium). Although they are mildly inhibitory, base cations also serve a role in reducing the toxicity of metals. For instance, Magnesium (Mg+2) can reduce the effect of toxic Manganese (Mn+2), because Mg is more soluble and more easily absorbed by microorganisms than Mn.
Table 5. Stimulatory and Inhibitory Concentrations of Base Cations in Anaerobic Digestion (from McCarty, 1964).
| Concentration mg/l | |||
|---|---|---|---|
| Cation | Stimulatory | Moderately Inhibitory | Strongly Inhibitory |
| Sodium, Na+ | 100 -- 200 | 3,500 – 5,500 | 8,000 |
| Potassium, K+ | 200 -- 400 | 2,500 – 4,500 | 12,000 |
| Calcium, Ca+2 | 100 -- 200 | 2,500 – 4,500 | 8,000 |
| Magnesium, Mg+2 | 75 -- 150 | 1,000 – 1,500 | 3,000 |
Acids
Low pH is usually a symptom rather than a cause of methane inhibition. The population of methanogens drops when a toxic material is added to a digester. The acid forming bacteria, which are generally hardier than methanogens, are less affected. More acid is produced than the weakened methanogens can digest, and as a result, pH goes down. A mature digester has a high alkalinity, or the ability to resist changes in pH, but there is a limit to alkalinity. If a digester receives a large, pH dropping slug of acidic material, acid forming bacteria will be promoted over methanogens, and the end effect is the same as introducing a toxic element.
A Co-Digestion Product is Toxic, Now What?
Let’s say you have completed an ATA test on a co-digestion product and it showed the substrate may inhibit biogas production. The first thing is not to despair. Anaerobic digesters have an undeserved reputation for being easily upset. The fact is, methanogenic organisms are no more sensitive to most chemicals than heterogenic aerobic microorganisms. The reason engineers familiar with sewage treatment plants “know” that digesters are prone to upset is, in sewage treatment plants at least, digesters are operated on the edge of their limits; whereas, other processes such as trickling filters and activated sludge units are “babied” and operated well within their biological envelopes. The truth is, digesters are frequently upset due to abuse rather than sensitivity to toxic materials. Given a long solids retention time, and large alkalinity, digesters can take many toxic upsets in stride.
The second thing is to understand is an ATA is an extreme test of toxicity. A large amount of potentially toxic material is introduced to a small amount of microbes with little external life support. The ATA is a good first brush at determining potential inhibition.
Once the ATA shows potential toxicity, the next step is to perform pilot scale testing. With longer retention times and constant feeding, the microbial communities may recover from the initial shock of an inhibitory agent. Or, the digester may develop resistance. Some chemicals may also bind with digester sludge or precipitate harmlessly in the digester.
Summary
The ATA test is the most accurate method of identifying substances toxic to biogas production in digesters. Common inhibitory materials include: antibiotics; cleaners, sanitizers, and disinfectants; heavy metals; materials containing ammoniacal nitrogen, sulfate, and sulfide; salty and acidic materials. Once an inhibitory material has been identified, it is best to pilot test in a small rector to determine if its toxic effect is temporary, or permanently crippling to gas production.
References
Blum, D.J, and R.E. Speece. 2012. A database of chemical toxicity to environmental bacteria and its use in interspecies comparisons and correlations. Research Journal of the Water Pollution Control Federation. 63(3):198-207.
Gartiser, S., E. Urich, R. Alexy, and K. Kümmerer. 2007. Anaerobic inhibition and biodegradation of antibiotics in ISO test schemes. Chemosphere. 66: 1839-1848.
ISO 13641-1 2003. Determination of inhibition of activity of anaerobic bacteria. Part 1: general test. International Organization for Standardization. Geneva, Switzerland.
ISO 13641-2 2003. Determination of inhibition of activity of anaerobic bacteria. Part 2: test at low biomass concentration. International Organization for Standardization. Geneva, Switzerland.
McCarty, P.L. 1964. Anaerobic waste treatment fundamentals. Part three: toxic materials and their control. Public Works. Nov 1964: 91-94.
McCarty, P.L., and R.E. McKinney. 1961. Salt toxicity in anaerobic digestion. Journal Water Pollution Control Federation. 33(4):399-415.
Moody, L.B., R.T. Burns, S.T. Sell, and G. Bishop. 2011. Using anaerobic toxicity assays to aid in co-substrate selection for co-digestion. Applied Engineering in Agriculture. 27(3): 441-447.
Sanz, J.L., N. Rodriquez, and R. Amils. 1996. The action of antibiotics on the anaerobic digestion process. Applied Microbiology and Biotechnology. 46:587-592.
Varel, V.H., and A.G. Hashimoto. 1982. Methane production by fermentor cultures acclimated to waste from cattle fed Monensin, lasalocid, salinomycin, or avoparcin. Applied and Environmental Microbiology. 44(6):1415-1420.
Zitomer, D.H., R.T. Burns, M. Duran, and D.S. Vogel. 2007. Effect of sanitizers, Rumensin, and temperature on anaerobic digester biomass. Transactions of ASABE. 50(5):1807-1813.
Douglas W. Hamilton, Ph.D., P.E.
Extension Waste Management Specialist
Biosystems and Agricultural Engineering
