Wheat Research at OSU 2018
- Jump To:
- Partnerships Enhance Wheat Research
- Perseverance Leads to Great Work
- Genetic Improvement and Variety Release of Hard Winter Wheat
- Wheat Pathology Research and Development of Disease-Resistant Germplasm
- Pest Resistance — Discovery and Introgression
- BCOA Resistance Introgression
- Gene Discovery, Transformation and Genomic Applications
- Understanding Genetic Variation on a Genomewide Scale
- Nitrogen-use Efficiency at the Genetic Level
- Wheat Breeding and Variety Development
- Wheat Variety Trials
- Testing methods
- Data Interpretation
- Additional information online
Partnerships Enhance Wheat Research
Partners in Progress – Oklahoma State University’s long-standing partnerships with the Oklahoma Wheat Commission and the Oklahoma Wheat Research Foundation are valuable assets for our wheat research and Oklahoma Cooperative Extension Service programs.
The partnerships provide more than partial funding for our research programs; they are sources of valuable feedback from producers to help keep our research programs focused and relevant.
They are truly one of the best examples of the Division of Agricultural Sciences and Natural Resources (DASNR) working in a cooperative relationship with commodity groups to achieve common goals. Partial funding for our research and Extension programs comes from wheat producers through the Oklahoma Wheat Commission and Oklahoma Wheat Research Foundation.
The Partners in Progress Wheat Research Report is one of a series of annual reports from DASNR highlighting research results and impacts of funded projects. This information is utilized throughout the year in educational programs and is distributed to Oklahoma wheat producers to keep them up to date on the latest research findings. The research contained in this report aims to meet the needs of Oklahoma wheat producers.
At the start of this report is a summary of accomplishments for fiscal year 2017-18 and follow up with detailed narratives that describe progress.
The long-term continuous support of our wheat research programs from the OWC and the OWRF has allowed our faculty to make significant progress toward the common goal of keeping Oklahoma wheat farmers competitive in regional, national and international markets. This support makes us truly partners in progress.
Keith Owens
Associate Vice President
Oklahoma Agricultural Experiment Station
Division of Agricultural Sciences and Natural Resources
Oklahoma State University
Perseverance Leads to Great Work
Few things are impossible to diligence and skill. Great works are performed not by strength, but by perseverance.—Samuel Johnson
The 2018 wheat harvest is complete, and the OSU Wheat Improvement Team (WIT) continues to focus on important research priorities within all areas of production.
The OSU public wheat research program continues to work to give wheat producers in the southern Plains greater opportunities when making seed selections that will have great agronomics and better options for marketability.
The top six planted wheat varieties in 2018, which also accounted for over 50 percent of the acreage in Oklahoma, came from OSU, according to a survey conducted by the United States Department of Agriculture, National Agricultural Statistics Service (USDA-NASS).
To carry on with these successes, the OSU Small Grains Variety testing program evaluates the yield potential and quality characteristics of over 25 commercially released wheat cultivars at about 20 locations throughout Oklahoma. In addition, the program evaluates 40 to 50 cultivars and experimental lines at five regional test sites to ensure that statewide tests are filled with the best-adapted cultivars. Data collected includes grain yield, disease resistance, response to fungicide application, adaptability to no-till production systems, high temperature sensitivity to germination, plant height, first hollow stem and heading data.
This year, we are proud of four new variety releases out of the OSU program — Showdown, Green Hammer, Baker’s Ann and Skydance. Each variety satisfies the critical need with end quality characteristics any miller or baker would be eager to work with. When it comes to dough strength and higher protein contents, the WIT remains focused on these important aspects that buyers seek. We also continue to focus on GrazenGrain® systems with many of our varieties. You will find more discussion about these new varieties on Page 30.
Releasing new varieties with different attributes continues to make us more competitive in the marketplace with both yield benefits and quality. The importance of creating varieties for maximum yield potential to make the producer more profitable is the main goal. However, it is also important to note the technologies funded to help release varieties that focus on better end-use value for the milling and baking industries. End-use quality attributes are highly regarded in the selections released through the OSU breeding program. This is extremely important when focusing on consumer needs.
In the breeding program at OSU, we examine and study the end-use quality characteristics that would benefit both our international and our domestic customers. That’s why we work to help farmers using our varieties capture more of the market. Quality starts with the seed placed in the soil. To have a good product for the end game, we must remember good quality also has to start from the beginning. We encourage soil testing that is available through your local county Extension office. We also encourage producers to look at the importance of nitrogen applications for increased-protein wheat that has better attributes for baking.
Focusing on some of these factors can help ensure good decisions are being made to deliver high-quality wheat. The OWC and the OWRF, along with OSU’s WIT and DASNR, continues to work to benefit both the producer and the customer. We move ahead by making great strides with the wheat research and Extension program at OSU, and we want to thank the producers for the support to keep these programs at the front of technology discovery and transfer. The OSU WIT prepares for planting by spending numerous hours on research with great diligence and skill. Nothing is impossible, and great works of our variety development program are performed with this perseverance — therefore we are glad to be partners in progress.
Mike Schulte, Executive Director
Oklahoma Wheat Commission
8820 Silver Hill Drive
Oklahoma City, OK 73132
Phone: 405-608-4350
Fax: 405-848-0372
Email: mschulte@okwheat.org
www.okwheat.org
Genetic Improvement and Variety Release of Hard Winter Wheat
Wheat Improvement Team
- Claimed the top six varieties for planted acreage in Oklahoma, according to an OWC-sponsored survey conducted by USDA-NASS in 2018 (WIT).
- Released four hard red winter varieties: Showdown, an upgrade for Bentley or Lonerider with very high yield potential; Green Hammer, a low-input option for downstate Oklahoma with high test weight and protein potential; Baker’s Ann, a unique combination of high yield potential in northern Oklahoma and anticipated high demand by millers and bakers; and Skydance, another low-input option featuring high test weight and protein with premium functionality centered on southwest Oklahoma (WIT).
- Placed 13 candidates under preliminary (six) or extended (seven) seed increase by
Oklahoma Foundation Seed Stocks. Two of these were confirmed to have strong resistance
to wheat streak mosaic, and 12 were moderately resistant or resistant to four of the
six diseases most frequently evaluated since 2014 (stripe rust, leaf rust, tan spot,
powdery mildew, wheat soil-bornemosaicorWSBM, and wheat spindlestreakmosaicorWSSM).
OCW04S717T-6W is highly resistant to all six diseases (Carver, Hunger).
OK1059018 reseln Billings/Duster
OK16D101089 OK12621/Bentley
OK16D101073 OK12621/Bentley
OK14124-2 NI04430/OK05303//Fuller
OK149132C CO06054/OK06029C
OK14P736W Australian sources/2*OK Bullet
OK12206-127206-2 Y98-912/OK00611W//OK03716W
OK13P016 Billings/Duster
OK14P212 OK01307/Duster//OK06822W
OK168512 Overley+/Fuller//2*CSU exptl.
OK168513 Overley+/Fuller//2*CSU exptl.
OK12912C-138407-2 N91D2308-13/OK03926C//OK03928C
OCW04S717T-6W CIMMYT seln/KS exptl.//KS91W047
- Evaluated 1,695 wheat experimental lines for field reaction to the wheat soil-borne mosaic/wheat spindle streak mosaic complex. A subset of 260 WIT experimental lines was further evaluated using the enzyme-linked immunosorbent assay to differentiate reactions to both viral diseases (Hunger).
- Evaluated 440 WIT experimental lines (12 nurseries) for seedling and adult plant reaction to leaf rust, 465 WIT experimental lines (13 nurseries) for seedling reaction to tan spot and powdery mildew, and 167 WIT advanced experimental lines for reaction to powdery mildew in field trials. Across replications, nearly 3,000 disease evaluations were made in the field in 2018 (Hunger).
- Identified seven of 22 WIT advanced experimental lines highly resistant to wheat streak mosaic (Hunger, Carver).
- Renovated part of the Small Grains Greenhouse Complex to comply with USDA-APHIS-PPQ standards to procure novel wheat germplasm from Hungary, Romania and Turkey (Hunger).
- Confirmed that Doublestop CL+ is moderately resistant to resistant to wheat streak mosaic (Carver, Hunger).
- Identified Tox A as the toxin nearly universally produced by Oklahoma isolates of Pyrenophora tritici-repentis, or the causal fungus of tan spot of wheat, the first key step in developing a system to screen for tan spot resistance (Hunger).
- Discovered two new powdery mildew resistance genes that can be widely used in the WIT variety development program and other wheat breeding programs, Pm223899 and Pm63 (Xu).
- Identified and characterized a new leaf rust resistance gene, Lr470121, providing a high level of resistance to leaf rust isolates collected in Oklahoma (Xu).
- Identified two wheat accessions that may carry resistance genes for dual protection against barley yellow dwarf, or BYD, and bird cherry-oat aphid, or BCOA (Xu).
- Produced first set of 133 experimental adapted lines with confirmed tolerance to BCOA, following field selection in 2018 for agronomic suitability among 416 lines (Giles, Zarrabi, Carver).
- Identified a new greenbug resistance source uniquely resistant to biotype G in Oklahoma, a highly virulent type of greenbug that can damage the vast majority of known sources of resistance in wheat (Xu).
- Developed diagnostic molecular markers for each of three candidate genes covering the targeted TaHf-A1 region in Duster that confers Hessian fly resistance (Yan).
- Confirmed the legitimacy of a genomic selection strategy targeting sedimentation volume adjusted for protein content, with even greater reliability than protein content itself or grain yield; disconcerting, however, was the unsuitability of incorporating traditional mixograph parameters into a genomic selection strategy due to very low predictability (Chen, Willyerd).
- Identified 55 single nucleotide polymorphisms in association with end-use quality traits and nine specifically for dough strength; interpretation of the genes impacted imply strong impact of disease on wheat quality (Chen, Willyerd).
- Evaluated 19 fungicide x fungicide rate combinations for control of wheat foliar diseases in field trials (Hunger).
- Provided in-season wheat disease updates to wheat growers, consultants, Extension educators and researchers via an electronic format (Hunger).
- Confirmed absence of Karnal bunt in Oklahoma wheat grain samples to allow Oklahoma wheat to move without restriction into the export market (Hunger).
After two strong decades of uninterrupted service, WIT is one of the longest-running research teams serving in any capacity at OSU. Faculty from three DASNR academic units form a complete team that combines fundamental and applied components of wheat research to propel a common cause — to advance Oklahoma’s wheat industry with development of improved varieties and dissemination of the know-how that best captures genetic potential.
The latest products of this charge came in the form of four new HRW wheat varieties. Showdown and Green Hammer extend the yield and quality performance of Bentley, Lonerider and Smith’s Gold in different parts of the state and beyond. Skydance and Baker’s Ann directly cater to an ever more discriminating market based on functional quality at competitive yields when produced in their targeted areas.
WIT scientists who received funding from the OWRF in 2017-2018 and reported their findings were Bob Hunger, wheat pathology research and development of disease-resistant germplasm; Xiangyang Xu, pest resistance discovery and introgression; Kris Giles and Ali Zarrabi, bird cherry-oat aphid, or BCOA, resistance discovery; Charles Chen, Karyn Willyerd and Liuling Yan, gene discovery and genomic technology; Brian Arnall, nitrogen-use efficiency; and Brett Carver, wheat breeding and variety development.
Recurring research projects in wheat disease diagnosis and evaluation, development of improved molecular tools to optimize breeding efficiencies, and variety development are common themes of WIT’s output. These must continue to sustain or build upon the advances made thus far. However, each year, WIT breaks new ground on several research fronts and uses this report to highlight exciting new discoveries that lay the foundation for future success.
Just a few of the advances reported here are:
- the higher frequency than expected of candidate lines offering strong adult-plant resistance to leaf rust,
- the emergence of new leaf rust and powdery mildew resistance genes with their ancillary markers,
- identification of bcoa tolerance within and outside the WIT pipeline and
- confirmation of useful levels of wheat streak mosaic resistance in statewide-adapted candidate lines.
WIT continues focusing on breakthrough research to understand how key traits important for Oklahoma — those which are complex and controlled by several genes — are regulated throughout the wheat genome, then eventually manipulated through a process called genomic selection. In the interest of financial and physical resources moving forward, this research will focus on quality traits for which the WIT has achieved indisputable success and recognition by the wheat industry.
WIT also has expanded its reach to more effectively serve wheat producers in the far western Oklahoma, having developed a smaller but highly targeted variety development program based at Goodwell as a part of the larger conventional breeding program. Thirteen HRW and HW candidate varieties remain at the center of WIT’s attention, and all but two of these are well adapted to far western Oklahoma.
In addition to advances in research, almost all WIT members engage with the agricultural community directly to enable wheat growers to make timely, effective management decisions.
Wheat Pathology Research and Development of Disease-Resistant Germplasm
Bob Hunger
Entomology and Plant Pathology
About 40 percent of the approximate 300,000 data points generated through an entire breeding cycle for one released variety can be attributed to disease reactions alone. Key diseases evaluated in 2018 included the wheat soil-borne mosaic/wheat spindle streak mosaic or WSBM/WSSM complex, leaf rust, powdery mildew, tan spot and barley yellow dwarf,or BYD. WIT will consider several other diseases, perhaps as many as eight to 10 more, in the final release of a variety. Table 1 presents the number of lines evaluated for reaction to the six diseases over the last 10 years, and Table 2 presents the number of lines evaluated from 1983 through 2018.
Field evaluations usually provide the most reliable indication of reaction to a disease. However, given the current size of the OSU variety development program or VDP, evaluation of experimental lines in a greenhouse setting allows evaluating many more lines than often possible in the field. Greenhouse testing also allows for consistent and reliable disease pressure and presence, which can be lacking in the field. Hence, greenhouse testing typically is conducted on many or all of the statewide, replicated breeding nurseries (22 were tested in 2018 for a total of 770 lines), whereas evaluation in field nurseries involves fewer lines in the more advanced nurseries.
Table 1. Number of wheat lines tested for disease reaction in the last 10 years. Data do not include ratings collected in breeding or Extension trials.
| Year | Testing Location | WSBM/WSSM | LR | YR |
|---|---|---|---|---|
| 2009 | Field | 1,500 | ||
| Greenhouse | 400 | |||
| 2010 | Field | 1,500 | ||
| Greenhouse | 400 | |||
| 2011 | Field | 1,400 | ||
| Greenhouse | 324 | |||
| 2012 | Field | 1,030 | ||
| Greenhouse | 427 | |||
| 2013 | Field | 2,410 | ||
| Greenhouse | 347 | |||
| 2014 | Field | 1,700 | ||
| Greenhouse | 466 | |||
| 2015 | Field | 1,500 | ||
| Greenhouse | 385 | |||
| 2016 | Field | 1,421 | 385 | |
| Greenhouse | 385 | |||
| 2017 | Field | 1,523 | ||
| Greenhouse | ||||
| 2018 | Field | 1,800 | ||
| Greenhouse | 770 | |||
| Total | Field & greenhouse evaluation | 15,784 | 4,235 | 385 |
| Year | Testing Location | PM | TS |
|---|---|---|---|
| 2009 | Field | ||
| Greenhouse | 400 | 400 | |
| 2010 | Field | ||
| Greenhouse | 400 | 400 | |
| 2011 | Field | ||
| Greenhouse | 67 | 262 | |
| 2012 | Field | 65 | |
| Greenhouse | 618 | 170 | |
| 2013 | Field | 197 | 95 |
| Greenhouse | 150 | 277 | |
| 2014 | Field | 21 | |
| Greenhouse | 141 | 411 | |
| 2015 | Field | ||
| Greenhouse | 115 | 385 | |
| 2016 | Field | ||
| Greenhouse | 385 | ||
| 2017 | Field | ||
| Greenhouse | 331 | 331 | |
| 2018 | Field | ||
| Greenhouse | 770 | 770 | |
| Total | Field & greenhouse evaluation | 3,254 | 3,907 |
| Year | Testing Location | STB | BYD |
|---|---|---|---|
| 2009 | Field | ||
| Greenhouse | |||
| 2010 | Field | ||
| Greenhouse | 400 | ||
| 2011 | Field | ||
| Greenhouse | 262 | ||
| 2012 | Field | 573 | |
| Greenhouse | 105 | ||
| 2013 | Field | 150 | |
| Greenhouse | 277 | ||
| 2014 | Field | 705 | |
| Greenhouse | |||
| 2015 | Field | 75 | 160 |
| Greenhouse | |||
| 2016 | Field | 145 | 145 |
| Greenhouse | |||
| 2017 | Field | ||
| Greenhouse | |||
| 2018 | Field | ||
| Greenhouse | |||
| Total | Field & greenhouse evaluation | 1,264 | 1,733 |
a WSBM/WSSM=complex of wheat soil-borne mosaic and wheat spindle streak mosaic; LR=leaf rust; YR=stripe rust; PM=powdery mildew; TS=tan spot; STB=Septoria tritici blotch; BYD=barley yellow dwarf.
Table 2. Summary of WIT lines evaluated for reaction to specific diseases from 1983 through 2018. Data do not include ratings collected in breeding trials or Extension trials.
| Disease | Year evaluations started | Evaluation locationa |
|---|---|---|
| WSBM/WSSMb | 1983 | GH |
| Field | ||
| Leaf rust | 1983 | GH – seedling |
| 2017 | GH – adult plant | |
| 1983 | Field | |
| Powdery mildew | 2000 | GH |
| 2011 | Field | |
| Tan spot | 2003 | GH |
| 2014 | Field | |
| Septoria tritici blotch | 2004 | GH |
| 2014 | Field | |
| Barley yellow dwarf | 2011 | Field |
| Spot blotch/common root rot | 2014 | GH |
| Total | 1983-2018 | GH |
| Field | ||
| 1983-2018 | GH + field |
| Disease | Year evaluations started | Number of lines evaluated |
|---|---|---|
| WSBM/WSSMb | 1983 | 500 |
| 36,261 | ||
| Leaf rust | 1983 | 21,691 |
| 2017 | 470 | |
| 1983 | 5,230 | |
| Powdery mildew | 2000 | 3,615 |
| 2011 | 1,630 | |
| Tan spot | 2003 | 3,756 |
| 2014 | 45 | |
| Septoria tritici blotch | 2004 | 1,200 |
| 2014 | 215 | |
| Barley yellow dwarf | 2011 | 505 |
| Spot blotch/common root rot | 2014 | 25 |
| Total | 1983-2018 | 31,257 |
| 43,886 | ||
| 1983-2018 | 75,143 |
a GH=greenhouse
b WSBM/WSSM=complex of wheat soil-borne and wheat spindle streak mosaic.
Disease assessments on the rise
Ideally, a combination of field and greenhouse evaluations are used to most reliably assess a line’s disease reaction. Such evaluations would not be possible without funds provided by the Oklahoma Wheat Research Foundation (OWRF); OWRF also has helped to fund attempts to expand evaluations. These ongoing and expanded evaluations have centered on three disease screening trials critical to variety release decisions.
A field nursery was established to evaluate BYD and powdery mildew. A field nursery located on the west side of Stillwater was used to rate the reaction of advanced WIT lines to powdery mildew and BYD. A variety susceptible to both BYD and powdery mildew (Pete) was planted in strips between breeder lines to facilitate incidence and severity of both diseases. To enhance the opportunity of infestation with aphids carrying the BYD virus, this nursery was planted in early September. To enhance the opportunity of powdery mildew infection, nitrogen was applied to the nursery at 100 percent of the soil-test recommended rate in the early fall, then again at 50 percent of the recommended rate in late winter, as high nitrogen favors powdery mildew. In 2018, BYD was not ratable, but powdery mildew was sufficiently severe so that seven advanced WIT nurseries (260 lines total) were evaluated. Combining greenhouse seedling ratings with field ratings provides a comprehensive and important evaluation of experimental lines for reaction to powdery mildew. Table 3 contains the results from the nursery on the west side of Stillwater. Note how the seedling ratings in the greenhouse consistently showed a higher level of susceptibility compared to field ratings, with Gallagher providing one obvious and familiar example. This discrepancy may be critically overlooked if relying strictly on seedling tests in the greenhouse.
Table 3. Comparison of seedling (greenhouse) versus adult plant (field) ratings for reaction to powdery mildew in 2018. Entries highlighted in boldface are candidates moving forward to 2019 nurseries (discussed in Carver’s report).
| Entry | Seedling ratinga | Field adult plant ratinga |
|---|---|---|
| Gallagher | I | R |
| Bentley | MS | MR |
| Lonerider | MR | MR |
| Stardust | I | MR |
| OK16D101004 | MR | R |
| OK16D101018 | I | R |
| OK16D101039 | I | R |
| OK16D101128 | MS | R |
| OK16D101136 | S | MR |
| OK16D101138 | MS | MR |
| OK16D101141 | MS | I |
| OK16D101157 | I | MR |
| OK16D101167 | S | I |
| OK16D101168 | I | MR |
| OK16D101191 | MS | MR |
| OK16D101199 | S | MR |
| OK16D101203 | MS | MS |
| OK16D101228 | I | MR |
| OK16D101237 | MS | I |
| OK16D101242F | R | R |
| OK16D101245 | MS | I |
| OK16DIB110 | MS | R |
| OK16DIB136 | MS | I |
| OK16D101072 | R | R |
| OK16D101073 | R | R |
| OK16D101075 | MR | R |
| OK16D101089 | R | R |
| OK16D101094 | MR | R |
| OK16D101099 | MR | R |
| OK16D101103 | R | R |
| OK16D101105 | MR | R |
| OK16D101113 | MR | R |
| OK16DIB127 | MR | R |
| OK16DIB128 | R | R |
| OK16DIB122 | MR | R |
| OK16D101304 | MR | MR |
| OK16D101314 | I | I |
| OK16D101315 | I | MR |
| OK16D101328W | I | I |
| OK16D101339 | S | I |
a S=susceptible; MS=moderately susceptible; I=intermediate; MR=moderately resistant; R=resistant
A post-vernalization greenhouse test for adult plant reaction to leaf rust was developed. A procedure to evaluate adult plant reaction to leaf rust in the greenhouse was successfully attempted in 2017. Hence, evaluation in 2018 expanded to include 13 breeding nurseries totaling 440 lines. In years when leaf rust pressure is too light to allow ratings under natural field conditions, this test fills a critical gap in the information needed to advance experimental lines in the VDP. An example of the results for screening one of the nurseries is presented in Table 4, showing the reaction of 45 advanced WIT lines expressed in seedlings (which is expressed during the entire life of a plant) as well as in adult plants after vernalization.
A field nursery to identify resistance to tan spot and Septoria is a priority. This has been an on-going attempt since 2012 and has met with only limited success, as indicated by the number of lines evaluated in the field for reaction to tan spot (Table 1). Currently, this project is taking a new direction. Recent research described below indicates tan spot is the primary leaf-spotting disease in Oklahoma. Hence, establishing a field nursery to evaluate tan spot reaction will be emphasized. A large area of wheat was planted with Billings (highly susceptible to tan spot) in 2018. During spring 2019, fields with a high incidence of tan spot in Oklahoma will be located and infested straw from those fields will be gathered and stored for placement in the nursery to provide the inoculum for tan spot.
Table 4. Comparison of seedling (greenhouse) versus adult plant ratings (greenhouse) for reaction to wheat leaf rust. Lines highlighted in orange exhibit adult plant resistance. Each set of backcross-experimental lines has the line in boldface above it as its recurrent parent.
| Entry | Seedling ratinga | Adult plant ratinga (post-vernalization) |
|---|---|---|
| OK10130 | S | S |
| OK15MASBx7 ARS 6-1 | Seg-S | MS |
| OK15MASBx7 ARS 6-2 | S | MS |
| OK15MASBx7 ARS 6-4 | S | S |
| OK15MASBx7 ARS 6-16 | S | S |
| OK15DMASBx7 ARS 6-4 | S | Seg-S |
| OK15DMASBx7 ARS 6-6 | S | MS |
| OK15DMASBx7 ARS 6-8 | S | S |
| Billings | MS | MR |
| OK15MASBx7 ARS 7-19 | MS | MR |
| OK15DMASBx7 ARS 7-17 | MS | MR |
| OK15DMASBx7 ARS 7-24 | MS | MR |
| OK15DMASBx7 ARS 7-41 | MS | S |
| OK15DMASBx7 ARS 7-57 | MS | MS |
| Gallagher | MR | R |
| OK15MASBx7 ARS 8-1 | I | Seg-MR |
| OK15MASBx7 ARS 8-2 | MR | R |
| OK15MASBx7 ARS 8-3 | S | MR |
| OK15MASBx7 ARS 8-5 | MS | R |
| OK15MASBx7 ARS 8-6 | I | I |
| OK15MASBx7 ARS 8-7 | MS | MR |
| OK15MASBx7 ARS 8-8 | MS | MR |
| OK15MASBx7 ARS 8-9 | I | MR |
| OK15MASBx7 ARS 8-12 | MR | R |
| OK15MASBx7 ARS 8-13 | Seg-S | MR |
| OK15MASBx7 ARS 8-14 | MR | R |
| OK15MASBx7 ARS 8-18 | Seg-MS | R |
| OK15MASBx7 ARS 8-19 | MR | R |
| OK15MASBx7 ARS 8-20 | MR | R |
| OK15MASBx7 ARS 8-23 | MS | R |
| OK15MASBx7 ARS 8-27 | MS | MR |
| OK15MASBx7 ARS 8-28 | MS | MR |
| OK15MASBx7 ARS 8-29 | Seg-S | |
| OK15MASBx7 ARS 8-31 | Seg-R | MR |
| OK15MASBx7 ARS 8-34 | MS | MR |
| OK15DMASBx7 ARS 8-59 | R | R |
| OK15DMASBx7 ARS 8-60 | MR | R |
| OK15DMASBx7 ARS 8-61 | MR | R |
| OK15DMASBx7 ARS 8-62 | R | R |
| OK15DMASBx7 ARS 8-66 | MR | R |
| OK12D22004-016 | MS | MR |
| OK15MASBx7 ARS 9-1 | MR | MR |
| OK15MASBx7 ARS 9-11 | Seg-R | I |
| OK15MASBx7 ARS 9-14 | R | MR |
| OK15DMASBx7 ARS 9-84 | R | MR |
a S=susceptible; MS=moderately susceptible; I=intermediate; MR=moderately resistant; R=resistant; Seg=segregating for reaction
Leaf spot diseases and wheat streak mosaic
Research conducted during 2016-2017 indicated tan spot, caused by the fungus Pyrenophora tritici-repentis (PTR), was the primary cause of leaf spot symptoms on wheat in Oklahoma. Subsequent research during 2017-2018 tested these isolates to determine their production of toxins that cause symptoms associated with tan spot, including chlorosis (yellowing) and necrosis (tissue death). There are three toxins produced by PTR, including Tox A, Tox B and Tox C. Tox A induces necrosis, and Tox B and C induce chlorosis (Figure 1). Research currently being planned will explore the use of the toxin (Tox A) to screen for reaction to tan spot rather than inoculating with the fungus. Use of the toxin would allow for a less expensive and less time-consuming technique to identify WIT lines resistant to tan spot.

Figure 1. Symptoms caused on wheat leaves by toxins produced by Pyrenophora tritici-repentis (causal fungus of tan spot of wheat). Necrosis (tissue death) produced on Glenlea and Katepwa is induced by Tox A; chlorosis (yellowing) produced on 6B365 is induced by Tox B or Tox C. Salamouni and 6B662 are wheat varieties resistant to all toxins, and hence, also resistant to tan spot.
Genetic markers are useful in identifying lines carrying disease- resistance genes, and this approach is used with wheat streak mosaic (WSM). However, confirmation by field testing is critical to ensure the resistance is expressed in the field at a sufficient level. Following negotiations with USDA-ARS and University of Nebraska-Lincoln in 2018, evaluation of WIT advanced lines was arranged to be conducted in western Nebraska on a contract basis. As depicted in Figure 2, the testing system in Nebraska provides for severe symptom expression and efficiently discriminates among lines resistant or susceptible to WSM. Results from 2018 indicated that seven of 22 WIT lines expressed a high level of resistance to WSM. These lines will continue to be evaluated for reaction to WSM and other agronomic traits, and they represent a significant step towards the development and release of a wheat variety resistant to this troublesome virus disease. Further discussion of WIT lines that may warrant candidacy for release is provided in Carver’s report.

Figure 2. Aerial view of the wheat streak mosaic screening nursery in Nebraska (top photo). The bottom photo is a closer view of individual wheat lines containing Doublestop CL+, Tomahawk (susceptible check), Mace (resistant check) and a WIT experimental line confirmed to carry the gene Wsm1 that will be further discussed in Carver’s report.
Official fungicide trials
Results for evaluating foliar fungicides in 2018 for their efficacy in controlling wheat foliar diseases are presented in Table 5. Rainfall was abundant from July through October (19.8 inches). November through January was drier (1.5 inches), but this rainfall, plus the rainfall prior to November was sufficient to sustain the wheat in this trial. Moisture received from February through May was 9.6 inches, which was drier than typical for the winter and spring. Most of Oklahoma also was dry during the winter and spring, and as a result, stripe rust was absent and leaf rust occurred only late (after the medium dough stage) in this trial. June was a wet month (6.0 inches), but harvest was not impeded by wet conditions.
Table 5. Effect of foliar fungicides on severity of powdery mildew, or PM, and leaf rust, yield and test weight, or TW, of Bentley wheat in Stillwater for 2017-2018.
| Treatment number Fungicidea; rate | GS appliedb | Date applied |
|---|---|---|
| 1. Non-sprayed check | --- | --- |
| 2. Trivapro; 9.4 oz/A FBd Trivapro; 9.4/A oz | 6 FB 10 | March 16 FB April 19 |
| 3. Tilt; 3.8 oz/A FB Trivapro; 13.7 oz/A | 6 FB 10 | March 16 FB April 19 |
| 4. Priaxor; 2 oz/A FB Nexicor; 7 oz/A | 6 FB 10 | March 16 FB April 19 |
| 5. Nexicor; 3.5 oz/A FB Nexicor 7 oz/A | 6 FB 10 | March 16 FB April 19 |
| 6. Nexicor; 3.5 oz/A FB Caramba 5 oz/A | 6 FB 10 | March 16 FB April 19 |
| 7. Tilt; 4 oz/A | 10 | April 19, 2019 |
| 8. Generic Folicur; 4 oz/A | 10 | April 19, 2019 |
| 9. Aproach Prima; 6.8 oz/A | 10 | April 19, 2019 |
| 10. Stratego Yield; 4 oz/A | 10 | April 19, 2019 |
| 11. Nexicor; 9 oz/A | 10 | April 19, 2019 |
| 12. Trivapro; 13.7 oz/A | 10 | April 19, 2019 |
| 13. Absolute Maxx; 4 oz/A | 10 | April 19, 2019 |
| 14. Absolute Maxx; 5 oz/A | 10 | April 19, 2019 |
| 15. Prosaro; 5 oz/A | 10 | April 19, 2019 |
| 16. Prosaro; 6.5 oz/A | 10 | April 19, 2019 |
| 17. Topguard EQ; 5 oz/A | 10 | April 19, 2019 |
| 18. Lucento; 5 oz/A | 10 | April 19, 2019 |
| 19. Trivapro; 13.7 oz/A | 6 | April 19, 2019 |
| LSD (P=0.05) |
| Treatment number Fungicidea; rate | PM (%)c April 13, 2019 | PM (%)c April 26, 2019 |
|---|---|---|
| 1. Non-sprayed check | 56 | 75 |
| 2. Trivapro; 9.4 oz/A FBd Trivapro; 9.4/A oz | 1 | 10 |
| 3. Tilt; 3.8 oz/A FB Trivapro; 13.7 oz/A | 5 | 5 |
| 4. Priaxor; 2 oz/A FB Nexicor; 7 oz/A | 9 | 15 |
| 5. Nexicor; 3.5 oz/A FB Nexicor 7 oz/A | 6 | 7 |
| 6. Nexicor; 3.5 oz/A FB Caramba 5 oz/A | 5 | 30 |
| 7. Tilt; 4 oz/A | 39 | 73 |
| 8. Generic Folicur; 4 oz/A | 36 | 73 |
| 9. Aproach Prima; 6.8 oz/A | 40 | 63 |
| 10. Stratego Yield; 4 oz/A | 39 | 53 |
| 11. Nexicor; 9 oz/A | 43 | 49 |
| 12. Trivapro; 13.7 oz/A | 43 | 60 |
| 13. Absolute Maxx; 4 oz/A | 53 | 76 |
| 14. Absolute Maxx; 5 oz/A | 63 | 56 |
| 15. Prosaro; 5 oz/A | 59 | 71 |
| 16. Prosaro; 6.5 oz/A | 63 | 69 |
| 17. Topguard EQ; 5 oz/A | 49 | 66 |
| 18. Lucento; 5 oz/A | 46 | 76 |
| 19. Trivapro; 13.7 oz/A | 1 | 10 |
| LSD (P=0.05) | 23 | 20 |
| Treatment number Fungicidea; rate | Leaf rust May 16, 2019 | Yield (bu/A) | TW (lb/bu) |
|---|---|---|---|
| 1. Non-sprayed check | 9 | 69 | 53 |
| 2. Trivapro; 9.4 oz/A FBd Trivapro; 9.4/A oz | 0 | 77 | 54 |
| 3. Tilt; 3.8 oz/A FB Trivapro; 13.7 oz/A | 0 | 80 | 53 |
| 4. Priaxor; 2 oz/A FB Nexicor; 7 oz/A | 0 | 79 | 53 |
| 5. Nexicor; 3.5 oz/A FB Nexicor 7 oz/A | 0 | 76 | 54 |
| 6. Nexicor; 3.5 oz/A FB Caramba 5 oz/A | 0 | 71 | 52 |
| 7. Tilt; 4 oz/A | 1 | 74 | 54 |
| 8. Generic Folicur; 4 oz/A | 3 | 73 | 53 |
| 9. Aproach Prima; 6.8 oz/A | 0 | 76 | 53 |
| 10. Stratego Yield; 4 oz/A | 0 | 79 | 54 |
| 11. Nexicor; 9 oz/A | 0 | 81 | 54 |
| 12. Trivapro; 13.7 oz/A | <1 | 72 | 54 |
| 13. Absolute Maxx; 4 oz/A | <1 | 76 | 54 |
| 14. Absolute Maxx; 5 oz/A | <1 | 75 | 54 |
| 15. Prosaro; 5 oz/A | <1 | 73 | 53 |
| 16. Prosaro; 6.5 oz/A | 4 | 74 | 53 |
| 17. Topguard EQ; 5 oz/A | <1 | 73 | 54 |
| 18. Lucento; 5 oz/A | <1 | 75 | 54 |
| 19. Trivapro; 13.7 oz/A | <1 | 72 | 53 |
| LSD (P=0.05) | 3 | NSd | NSd |
a Plus 0.125% Induce (volume by volume) for treatments 13-16; plus 0.25% Induce (volume
by volume) for treatments 2-6, 11, 12, 17-19.
b GS (growth stage) is reported according to Feekes’ scale, where GS 6=first node
detectable at base of main tiller; GS 10=head in boot but not emerging.
c PM=powdery mildew; rated on lower leaves on April 13 and on lower leaves on April
26.
d FB=followed by; NS=no statistical significance.
Symptoms indicative of BYD were present in the spring, and spotty stunting due to BYD was observed. This virus disease may have affected yield to a slight extent. Powdery mildew reached a severity of 75 percent on lower to mid-canopy leaves by late April. Light and scattered powdery mildew also was observed on flag leaves and on wheat heads into May but did not reach ratable levels. Treatments that received an early fungicide application on March 16, 2018, showed significantly lower powdery mildew severity compared to treatments that received a single fungicide application on April 19. Leaf rust was just beginning to establish in mid-May when plant senescence started to occur. Grain yield varied from 69 bushels per acre (nontreated check) to 81 bushels per acre. Test weight varied from 52 to 54 pounds per bushel. Grain yield from fungicide treatments (75 bushels per acre) was not significantly greater than the yield of the nontreated control (69 bushels per acre). Treatments receiving two fungicide applications had an average yield (77 bushels per acre) that did not significantly exceed the average yield of treatments receiving a single fungicide application (75 bushels per acre).
On two final notes, novel wheat germplasm was exchanged again in 2018 with the national wheat breeding programs in Hungary, Romania and Turkey. In order to receive this germplasm, the initial grow-out must be conducted in a facility approved by the USDA-APHIS-PPQ. Hence, a greenhouse room in the OSU Small Grains Greenhouse complex was renovated to accommodate those conditions. This germplasm is used in crossing with locally adapted wheat varieties, with the purpose of introgressing novel and useful traits into the OSU wheat pipeline. Expanding the OSU wheat genetic pool in this manner is a constant goal.
Timely electronic updates on the status of wheat diseases were provided to wheat producers, Extension educators and others involved with wheat. The 2018 Oklahoma wheat crop was tested (15 samples from eight counties) for the presence of Karnal bunt. Results from this testing were used to certify that Oklahoma wheat was produced in areas not known to be infested with Karnal bunt, which allows Oklahoma wheat to move freely into the export market.
Pest Resistance — Discovery and Introgression
Xiangyang Xu
USDA-ARS
Wheat, Peanut and Other Field Crops Research Unit
This part of the WIT is dedicated to using multiple tools from several disciplines, including wheat pathology and entomology, molecular genetics and wheat pre-breeding to diversify and fortify the germplasm base on which WIT’s variety development pipeline depends. Gene introgression is a highly worthy but time-consuming process that often involves multiple steps to reach a commercial product. A research project may be mentioned here but go unmentioned in a subsequent Partners in Progress report, as gene introgression plays out over several breeding cycles.
Genetics behind aphid resistance
Greenbug and BCOA are important wheat pests, and resistance sources are urgently needed for wheat improvement. One particular accession described previously in Partners in Progress, TA3516, consistently exhibited resistance to greenbug and BCOA; thus a recombinant inbred line (RIL) population derived from the cross TA3516 x Bainong418 was developed to identify the responsible genes.
This RIL population of 245 F6 experimental lines was sequenced, producing 4,908 high-quality, single-nucleotide polymorphism (SNP) markers. Currently, BCOA resistance of the RILs is being evaluated using a method reported previously, and greenbug biotype E responses were to be assessed in early January 2019. In spring 2019, this data collection phase is expected to be complete. QTLs for BCOA resistance will then be identified, as will the gene for greenbug resistance in TA3516. SNPs closely linked to the targeted QTLs or gene will be converted to PCR-based, high-throughput Kompetitive Allele Specific PCR (KASP) markers for marker-assisted selection of desired progeny from crosses already made with WIT elite lines.
Seeking new aphid-resistant sources
Screening continued in 2018 for BCOA resistance within a large set of about 7,000 U.S. wheat accessions. Utah No. 101 A149 and Harvest Queen 2433 may feature novel BCOA resistance. Utah No. 101 A149 and Harvest Queen 2433 are winter and spring wheat lines, respectively, and both showed high resistance to BYD in previous studies. The goal is to introgress the BCOA/BYD resistance of Utah No. 101 A149 to WIT elite lines. Also, BCOA resistance from two accessions featured in the 2017 Partners in Progress report, Osiris and Ghund Hosa, is being backcrossed into WIT variety Stardust.
Greenbug is a major vector of the BYD virus in the U.S. After identifying a new greenbug resistance gene Gb595379 in the line PI 595379-1 in 2017, U.S. germplasm continued to be screened for new resistance sources. A wheat accession with unknown origin, YS, is resistant to greenbug biotype E, and the underlying resistance gene was mapped to a genomic region near Gb3 on the long arm of chromosome 7D. YS is likely to carry the Gb3 gene, which is susceptible to greenbug biotype G. However, three YS plants were found to be highly resistant to greenbug biotype G, while all others were susceptible. Therefore, these three plants may carry a new or additional resistance gene(s). The resistance gene(s) in these plants will be characterized next.
New powdery mildew resistance genes
Powdery mildew is an important foliar disease caused by Blumeria graminis f. sp. tritici (Bgt), and the major powdery mildew resistance genes deployed in the Great Plains, such as Pm3 and Pm17, have lost effectiveness in the U.S. Therefore, identifying new powdery mildew resistance genes is essential for sustainable improvement of wheat varieties. With the support of OWRF, two new powdery mildew resistance genes, Pm63 and Pm223899, were found to confer high resistance to Bgt isolates in the Great Plains. Pm63 was identified in Iranian landrace PI 628024 and was located to a 13.1 Mb interval on the long arm of chromosome 2B, spanning from 710.3 to 723.4 Mb in the Chinese Spring reference sequence (Figure 3). Pm63 was 1.1 cM proximal to STS marker Xbcd135-2 and 0.6 cM distal to SSR marker Xstars419. Both Xbcd135-2 and Xstars419 have the potential to tag Pm63 in breeding populations.
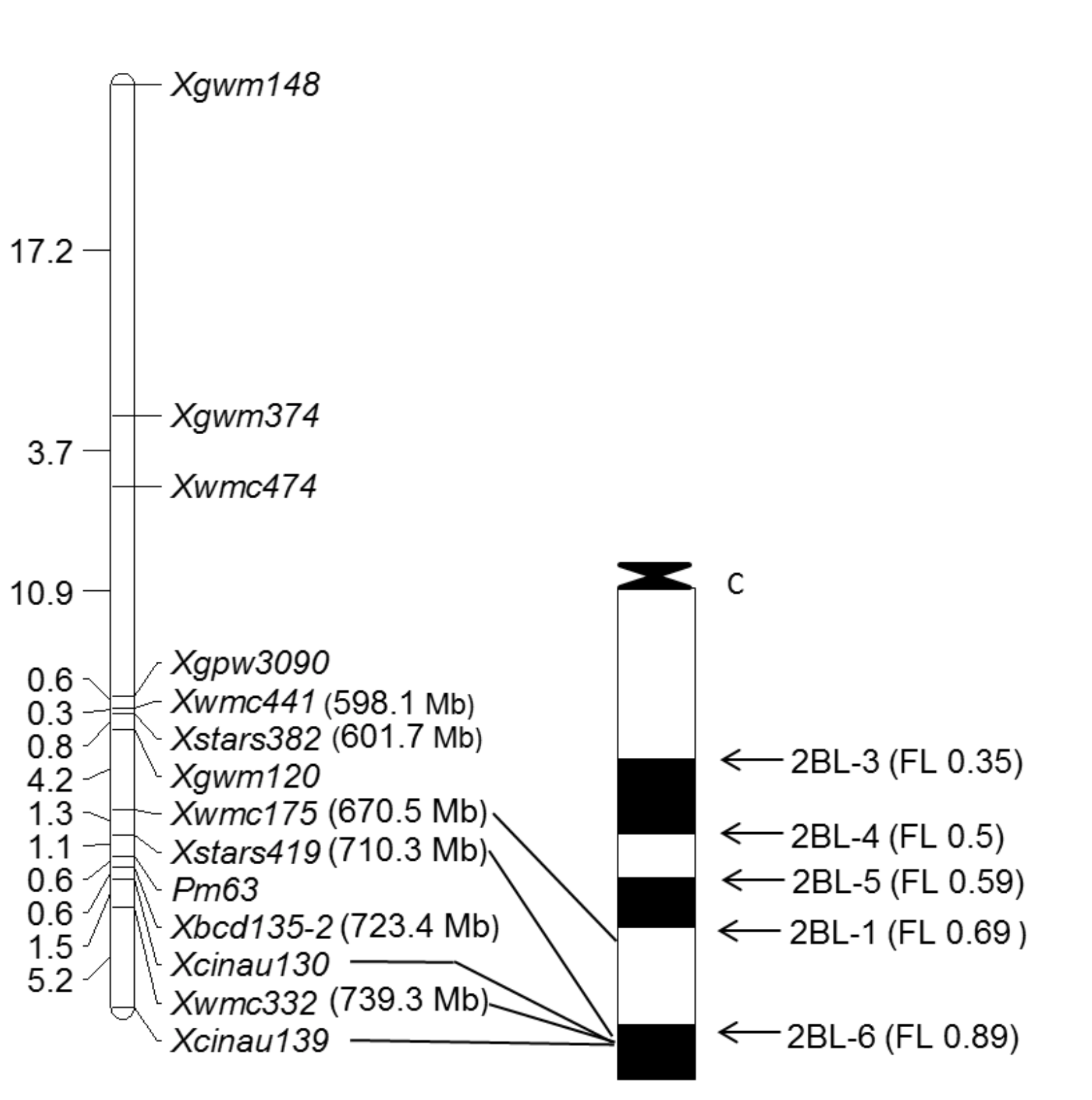
Figure 3. Linkage (left) and physical bin maps (right) for Pm63. Marker loci names are shown at the right of the linkage map, and genetic distances are shown in cM on the left. The physical positions of some markers on the Chinese Spring reference assembly IWGSC RefSeq v1.0 are enclosed by parentheses. Molecular markers flanking Pm63 are connected to their appropriate physical bins. The breakpoint of each Chinese Spring deletion line is shown with an arrow, and the corresponding fraction length (FL) value is given in the following parentheses.
Pm223899 is a recessive gene identified in Afghanistan wheat landrace PI 223899 and was mapped to an interval of about 831 Kb in the terminal region of the short arm of chromosome 1A (Figure 4), spanning from 4,504,697 to 5,336,062 bp of the Chinese Spring reference sequence. Eight genes were predicted in this genomic region, including TraesCS1AG008300 that encodes a putative disease resistance protein RGA4. Pm223899 was flanked proximally by SSR marker STARS333 (1.4 cM) and distally by the Pm3 locus (0.3 cM). Pm3b-1 and Xstars333 have the potential to tag Pm223389 in breeding populations. Both Pm63 and Pm223899 confer a high level of resistance to Bgt isolates collected from the Great Plains, and introgression of them into WIT elite lines is currently underway.
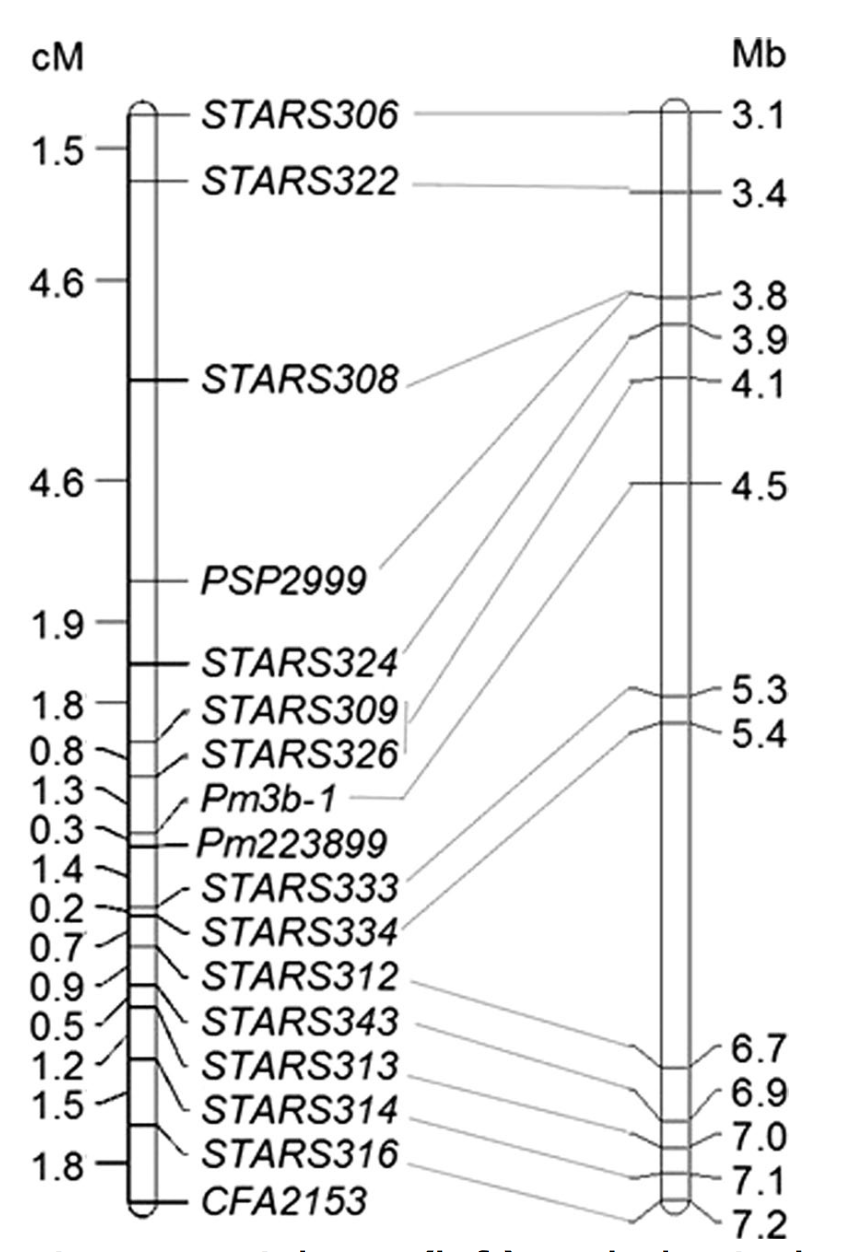
Figure 4. Linkage (left) and physical maps (right) for Pm223899. Marker loci names are shown at the right of the linkage map, and genetic distances are shown in cM on the left. The physical positions of molecular markers are given at the far right of the physical map.
Characterization of a novel leaf rust resistance gene
Leaf rust, caused by Puccinia triticina (Pt), is the most common and widespread rust disease in wheat. These Pt races evolve rapidly in the southern Great Plains, and leaf rust resistance genes often lose effectiveness shortly after their deployment in wheat production. PI 470121, an experimental line developed by the University of Zagreb in Croatia, showed high resistance to Pt races collected from Oklahoma, suggesting that PI 470121 is a potential leaf rust resistance source for the southern Great Plains. Genetic analysis based on the F2 population and F2:3 progeny derived from the cross PI 470121 x Stardust indicated that PI 470121 carries a dominant seedling resistance gene, designated Lr470121. Linkage mapping delimited Lr470121 to a genomic region of approximately 4.8 Mb, spanning from 60.80 Mb (Xstars477) to 65.65 Mb (Xstars480) in the Chinese Spring reference sequence (Figure 5). Lr470121 was 0.6 cM distal to Xstars480 and 0.9 cM proximal to Xstars477. SSR markers Xstars480 and Xstars477 have the potential to tag Lr470121 in breeding populations. In addition, PI 470121 also carries the adult-plant resistance gene Lr34. The simultaneous introgression of Lr470121 and Lr34 into adapted germplasm is feasible using marker-assisted selection and may lead to durable leaf rust resistant varieties.
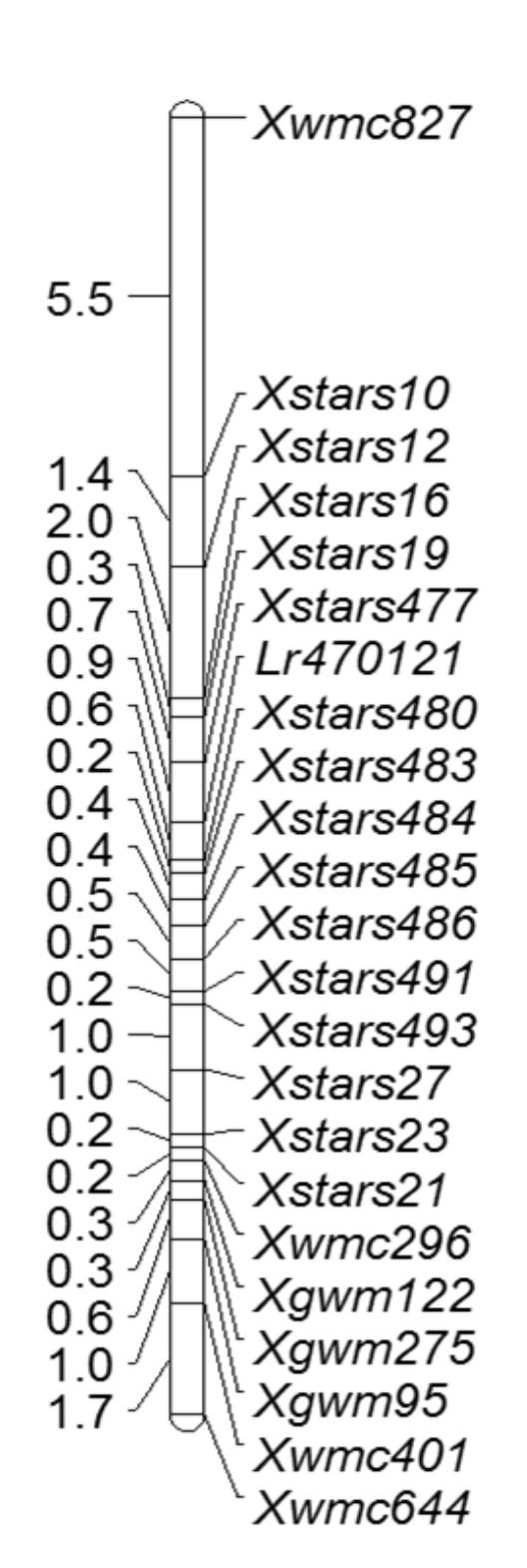
Figure 5. A linkage map for Lr470121. Marker loci names are shown at the right of the linkage map, and genetic distances are shown in cM on the left.
BCOA Resistance Introgression
Kris Giles
Ali Zarrabi
Entomology and Plant Pathology
The long-term goal to identify breeding populations enriched for resistance to BCOA infestations is coming into view. For the 2017-2018 cycle, a validation trial was conducted on susceptible varieties to confirm that the phenotyping protocol, which was well described in previous reports, accurately measures plant damage over time and that the BCOA aphid colony source population has remained virulent. Plant damage results were consistent with previous evaluations on susceptible entries, and the BCOA colonies remain virulent (Figure 6). However, in an effort to maintain current wild-type virulence present in Oklahoma wheat fields, introduction of field-collected BCOA into the continuing laboratory colonies is planned each year from multiple locations in Oklahoma.

Figure 6. Plant damagefromBCOA feeding in controlled-environment assays were consistent to previous evaluations on susceptible entries, such as Jagger (left).BCOA source colony maintainedbyZarrabi and Giles (right).
In addition, the screening results that identified six F5 populations from the variety development pipeline with fair-to-excellent levels of resistance to BCOA were used to select F5:6 lines that were included in 2018 field trials conducted by Carver. See Page 29 for more on those trials. Progeny from the two most promising populations (162056-055 and 162052-038) were screened again and confirmed to be tolerant to BCOA feeding, then used to build a crossing block in the 2018 greenhouse cycle. Going forward, WIT will validate resistance to BCOA from entries that were included in field trials, and those entries with BCOA resistance and desirable agronomic traits will be used in crossing schemes designed to improve variety performance. Lines with the validated BCOA resistance have a high probability of direct commercialization, pending statewide yield and quality trials in progress currently.
Gene Discovery, Transformation and Genomic Applications
Liuling Yan
Plant and Soil Sciences
Validating and tracking Hessian fly resistance
Hessian fly (HF) is one of the most destructive pests of U.S. wheat, and the Great Plains (GP) biotype is the most prevalent in the southern Great Plains. More than 16 genes for resistance against wheat diseases have been cloned, allowing a better understanding of the molecular genetic mechanisms of wheat-disease interactions and more effective utilization of disease resistance genes in breeding populations. However, no gene has been cloned for resistance against any insect pest of wheat.
In previous work of this laboratory, a major HF resistance gene unique to Duster was mapped to the short arm of chromosome 1A in the TaHf-A1 region, using genotyping-by-sequencing (GBS) markers. In current studies, 4,500 conventional progenies of Billings x Duster were screened for crossover events to narrow the targeted genomic region to 169 kb (as last reported, this region was delimited to 180 kb), where only three candidate genes exist based on the genome sequence of Chinese Spring. The results have provided an excellent opportunity to clone the first wheat gene for insect resistance.
Diagnostic molecular markers were developed for each of the three candidate genes covering the targeted TaHf-A1 region in Duster. With effective markers, this key characteristic of Duster can be tracked and introgressed into future Oklahoma wheat varieties, but better markers need to be developed for larger wheat breeding programs like this one to improve selection efficiency. WIT is pleased to report that USDA-NIFA recently committed in November 2018 to further fund this OWRF-supported research to produce a practical high-throughput genotyping system. Such a marker system will allow WIT and other breeding programs in Kansas and Nebraska to more efficiently breed with the unique source of HF resistance from Duster.
Identification and utilization of unique sequences within a grain yield QTL
One of WIT’s overarching research objectives is to identify genes controlling yield and yield components and incorporate desirable yield genes into novel winter wheat varieties. As another valuable trait in Duster, the QYld-osu-1B region, a quantitative trait locus, or QTL, on chromosome 1BS (short arm) was found to increase grain yield 20 percent to 25 percent compared with the same genetic locus in Billings, another OSU variety with high yield potential due to contributions from other yield genes. In the WIT breeding program, Duster or its offspring or grand-offspring appear in the pedigrees of about 25 percent of all experimental lines. Thus identifying, validating and continual tracking of the candidate gene(s) for QYld-osu-1B constitute the single most important molecular target for improving grain yield in Oklahoma.
The major gene in the QYld-osu-1B region has been identified for grain yield in an approximate 25 Mb region on chromosome 1BS in Duster. Unique sequences were discovered for three genes (CLP, ZFP4 and NMP) in Duster, relative to sequences in the comparable region in Billings, 2174 and Chinese Spring. Duster also possessed the dominant allele for eight genes, compared to Billings (Table 6). Though this genomic region has been notoriously recalcitrant to recombination — a genetic oddity by itself — 32 recombinant events were discovered among 6,406 gametes in the targeted QYld-osu-1B region. These recombinant progeny provide the fuel for more precise mapping of the chromosomal location of this near-mystical yield gene in wheat.
Table 6. Unique sequences identified in the QYld-osu-1B region of Duster and polymerase chain reaction, or PCR, markers mapped in a Billings x Duster doubled haploid population.
| Physical distance (Mb) | Gene name | Marker |
|---|---|---|
| 0.9 | TSSR5 | Dominance for the Duster allele |
| 1.2 | TSSR7 | Dominance for the Duster allele |
| 1.2 | RFP | Dominance for the Duster allele |
| 1.3 | TSSR8 | Dominance for the Duster allele |
| 1.4 | TSSR9 | Dominance for the Duster allele |
| 1.4 | PLT(F1R2) | Dominance for the Duster allele |
| 2.4 | TSSR17 | Dominance for the Duster allele |
| 3.5 | GBS SNP | GBS12138 |
| 4.7 | CLP | Unique sequences in Duster |
| 5.8 | Pm3-B1 | Pm3-1321-F2/R3,1920bp |
| 10.1 | ZFP4 | Unique sequences in Duster |
| 15.7 | WNK | No difference between Duster and Billings |
| 17.3 | XCP | No difference between Duster and Billings |
| 17.7 | NMR | Unique sequences in Duster |
| 17.8 | PGR5(UN) | SNPs between Duster and Billings |
| 18.4 | NAK | SNPs between Duster and Billings |
| 20 | OXR | No difference between Duster and Billings |
| 21.7 | TIM | No difference between Duster and Billings |
| 22.1 | OEP | No difference between Duster and Billings |
| 25.2 | WCK | SNPs between Duster and Billings |
| Physical distance (Mb) | Gene name | Comment |
|---|---|---|
| 0.9 | TSSR5 | A dominant marker |
| 1.2 | TSSR7 | A dominant marker |
| 1.2 | RFP | A dominant PCR marker, mapped |
| 1.3 | TSSR8 | A dominant PCR marker, mapped |
| 1.4 | TSSR9 | A dominant marker |
| 1.4 | PLT(F1R2) | A dominant PCR marker, mapped |
| 2.4 | TSSR17 | A dominant marker |
| 3.5 | GBS SNP | STARP |
| 4.7 | CLP | dCAP marker, mapped |
| 5.8 | Pm3-B1 | Billings allele dominant, mapped |
| 10.1 | ZFP4 | PCR marker, mapped |
| 15.7 | WNK | |
| 17.3 | XCP | |
| 17.7 | NMR | F8/R3, A=420, B=400 |
| 17.8 | PGR5(UN) | F4/R4+MseI |
| 18.4 | NAK | F2/R2+FspI |
| 20 | OXR | |
| 21.7 | TIM | |
| 22.1 | OEP | |
| 25.2 | WCK | dCAP marker, mapped |
Additionally, PCR markers were developed for the unique sequences in Duster according to SNPs between Duster and Billings. The same primers were used to run PCRs with Duster and Billings, and PCR products with expected sizes were directly sequenced, confirming a single copy of the PCR products. PCR products were distinguished between two alleles by using appropriate restriction enzymes for digestion. Four of the PCR markers are shown in Figure 7. The PCR markers corresponding to unique sequences in Duster were used to screen up to 200 hard winter wheat lines from the southern Great Plains, but no line was found to have the same allele as Duster. Hence, Duster is a unique cultivar that can be used to increase grain yield, as already proven by conventional selection and breeding, but WIT expects this genetic resource to have even greater impact and utility once the optimal marker(s) are identified.
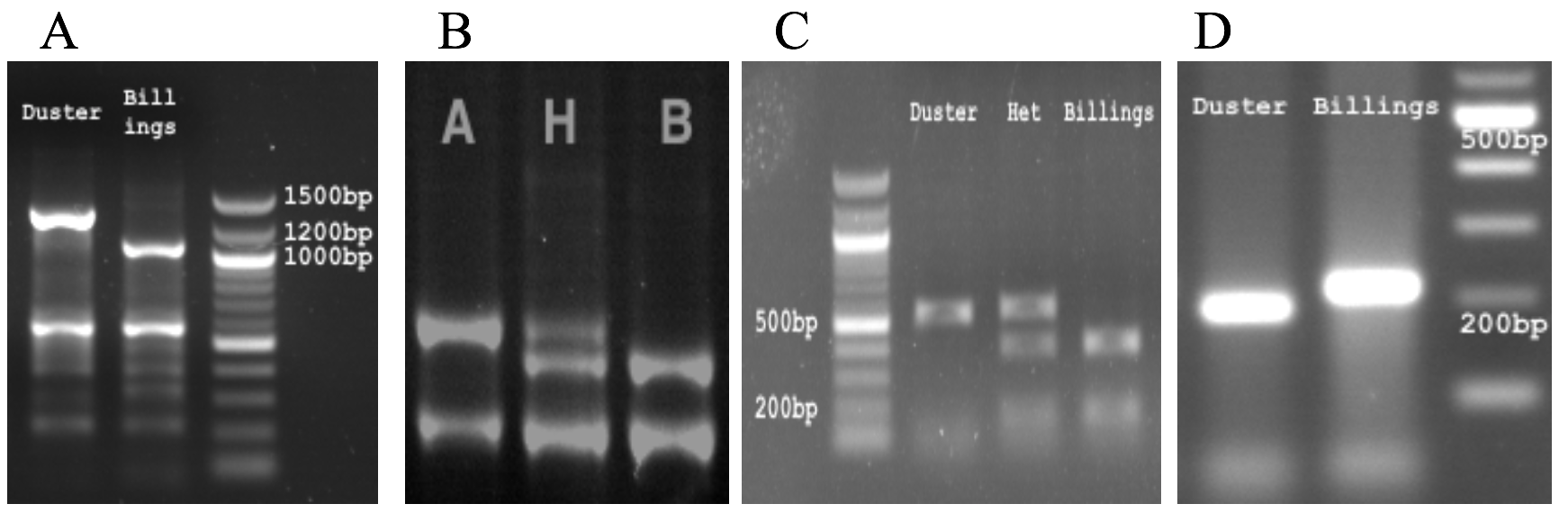
Figure 7.PCR markers for QYld.osu-1BS: A)PLT-F2R2, B) GBS12138, C) ZFP4-F4R4, D)WCK,dCAP-1B-3.
Developing KASP markers for Oklahoma-relevant genes
Over the course of OWRF funding for this part of WIT, more than 10 genes were identified that play critical roles in plant development, adaptation and pest resistance. These include VRN-A1, VRN-D3 and PPD-D1 that regulate heading date; HOX1 and ANR1 that regulate reproductive development and nitrogen-use efficiency; Lr34, Pm3a, Yr17 and Xa21-5A that confer resistance against foliar diseases; MFT-A1 that confers high-temperature germination sensitivity; and ALMT1 that confers tolerance to acidic soils. PCR markers for these functional genes were utilized in OSU breeding populations in previous years. Emphasis has now switched to converting the available PCR markers into KASP assays for greater selection efficiency. These high-throughput KASP assays will be used to track favorable alleles for some, if not all, of the genes designated above, in breeding populations most relevant to Oklahoma.
Understanding Genetic Variation on a Genomewide Scale
Charles Chen
Karyn Willyerd
Biochemistry and Molecular Biology
Duster and Billings historically are important winter wheat varieties for both yield and end-use quality in the southern Great Plains. After intercrossing these two landmark OSU varieties, a DH population of 282 lines was generated hereafter called Buster, providing a segregating population in which genetic mechanisms responsible for important and economic phenotypes can be disclosed in detail. The previously reported SNP dataset for Buster, built from genotyping-by-sequencing (GBS) and exome capture technologies, was re-anchored to generate 213,940 SNPs in this population. Genomewide association studies, or GWAS, and QTL mapping were performed further in 2018 to identify genomic regions associated with traits important to the Oklahoma wheat industry.
Genomewide association analysis – Buster yield and quality
Utilizing the Buster genomic resource as the genetic backdrop, trait architecture has come into better view for grain yield and several key quality parameters, including wheat and flour protein content, kernel hardness and gluten strength according to a sodium dodecyl sulfate, or SDS, sedimentation test. Using a threshold for significance of p<5e-4, GWAS revealed a total of 145 SNPs for grain yield, 13 and 18 SNPs, respectively, for wheat and flour protein; 9 SNPs for SDS sedimentation volume; and 18 and 23 SNPs, respectively, for single-kernel characterization system, or SKCS, and near-infrared reflectance, or NIR, measurements of hardness index (Figure 8). Significant SNPs associated with grain yield were prominent on chromosomes 1BS and 2DL, whereas the quality traits demonstrated lower levels of significance more broadly across the genome. The high SNP frequency associated with grain yield on chromosome 1BS may not be surprising given the importance of this region to yield segregation among Billings x Duster progeny discussed in Yan’s report.
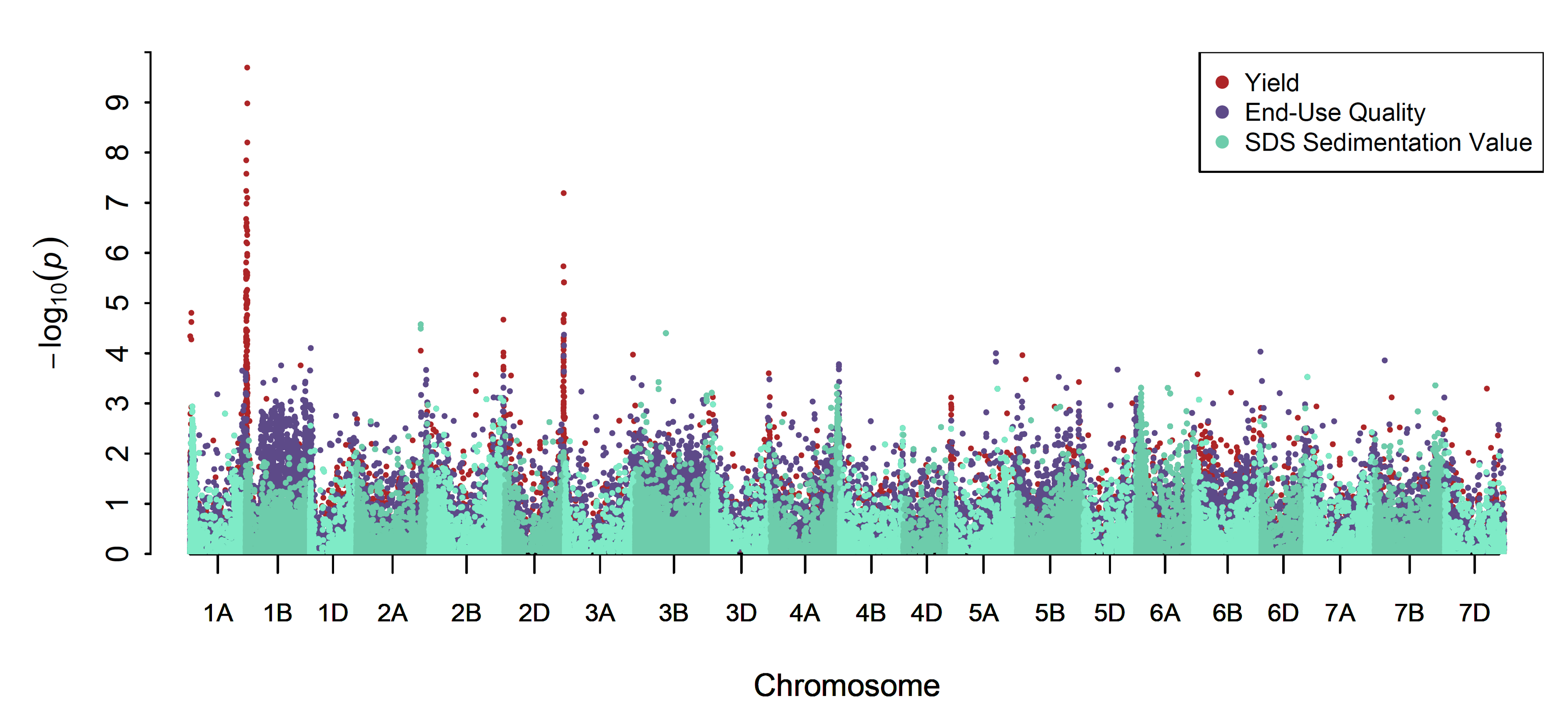
Figure 8. Genomewide association study of the Buster doubled haploid population for grain yield and end-use quality traits in which sedimentation volume has been partitioned out. Chart shows the results of a best linear unbiased prediction (BLUP) model to remove year-to-year environmental variation. Genetic hot spots can be detected for grain yield on chromosomes 1BS and 2DL, whereas genetic density was more evenly dispersed across the genome for quality traits.
Figure 9 singles out chromosome 1BS, where genetic correlation of multiple phenotypes was highly significant. As expected, wheat and flour protein traits were closely correlated, as were both measurements of hardness index. Kernel hardness estimated by SKCS is directly proportional to force by crushing individual kernels, whereas kernel hardness estimated by NIR is a function of ground particle size, in which harder kernels produce larger particle size. SNPs for SDS sedimentation values relating to gluten strength were not significant on chromosome 1BS. Indeed, sedimentation values were adjusted for protein differences so that any differences in gluten strength were more closely tied to differences in inherent protein quality, or swelling potential of a flour suspension, rather than protein quantity.
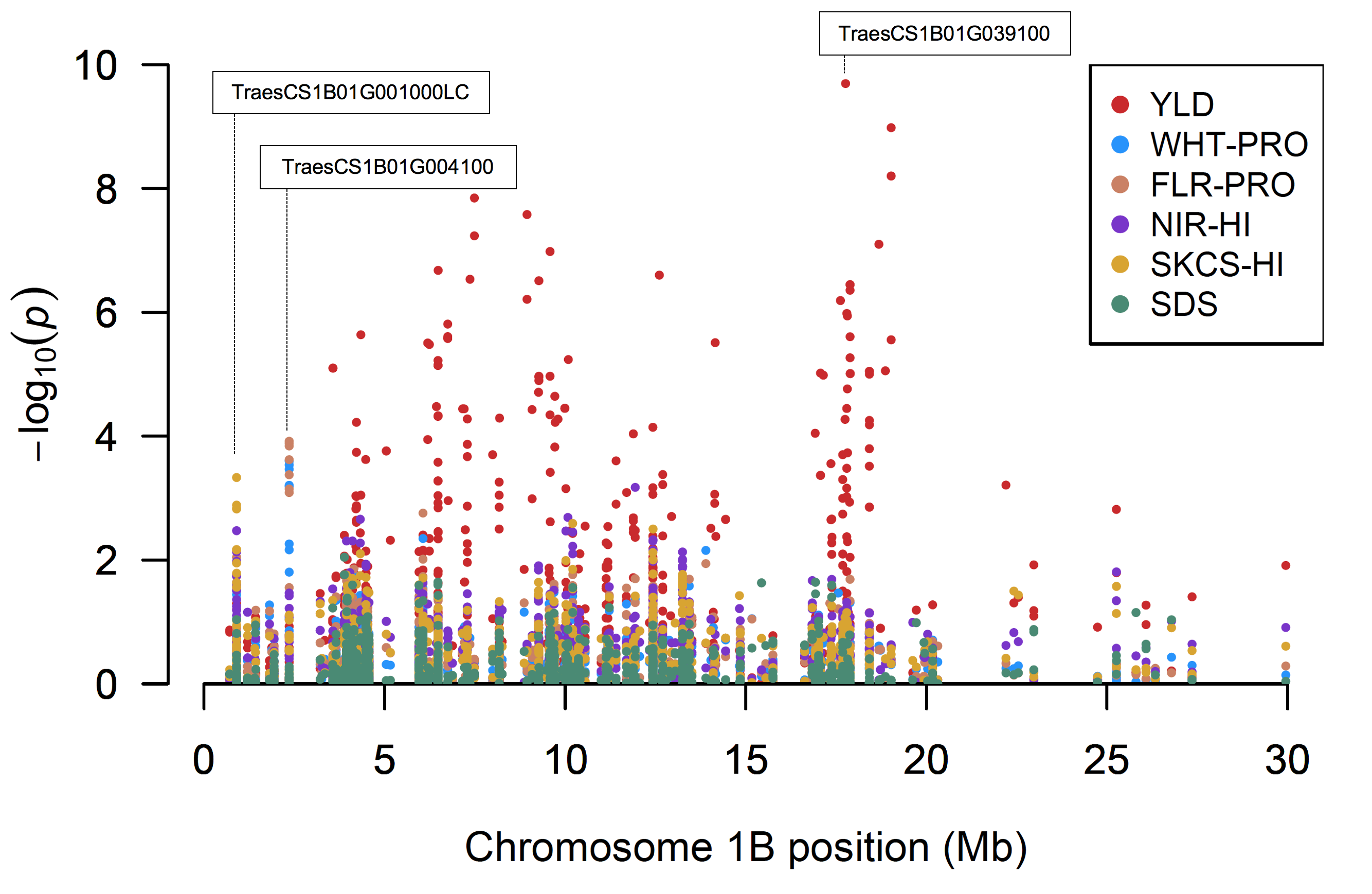
Figure 9. Examination of significant single nucleotide polymorphisms present on chromosome 1BS. End-use quality parameters are individually characterized. YLD = grain yield; WHT-PRO = wheat protein content; FLR-PRO = flour protein content; HI = hardness index based on near-infrared reflectance, or NIR, spectroscopy or on single-kernel characterization system, or SKCS; SDS = sodium dodecyl sulfate sedimentation volume adjusted for as-is flour protein content.
Within this 30 Mb region on chromosome 1BS, one significant SNP for SKCS hardness index (S1B_901595) was associated with an International Wheat Genome Sequencing Consortium, or IWGSC, low-confidence gene (TraesCS1B01G001000LC). RNA sequencing of selected DH individuals and parental lines indicated transcript expression at this location, thus confirming the presence of this gene in Duster. Although most were of low significance, 23 SNPs were identified within 100 bp of a single exon of this putative gene. Lowering the threshold of significance to p < 5e-3, three additional SNPs for SKCS hardness index (S1B_901534, S1B_901597 and S1B_901602) and one for NIR hardness index (S1B_901539) were found close to one another. A homology search of this gene sequence using NCBI’s Basic Local Alignment Search Tool (BLAST) found 97 percent identity to the putative disease resistance protein At1g50180 in Aegilops tauschii (accession XM_020313144).
Seven significant SNPs (S1B_2357396 to S1B_2357444) represented flour protein content, five of which were shared with wheat protein content. This region produced a haplotype frequency of approximately 65 percent Duster and 35 percent Billings genotypes across the DH population, with a positive mean effect of 0.34. The top 25 percent of phenotypic values for protein content contained 30 individuals exhibiting the minor haplotype. All seven SNPs were close and mapped to a single IWGSC gene annotation (TraesCS1B01G004100) identified as a receptor-like protein kinase (Figure 9). These proteins are generally regarded as immunoproteins and are implicated in the regulation of biotic and abiotic stress responses, as well as plant growth and development.
Upon further investigation, BLAST results revealed this protein sequence is identical to a previously characterized Triticum aestivum gene Snn1 (accession KP091701). Identified in a multiparent wheat population on chromosome 1BS, this locus reflects the sensitivity or resistance to effector proteins secreted by the fungal pathogen Parastagonospora nodorum. This pathogen is the causative agent of Septoria glume blotch also known as Septoria nodorum blotch, a common disease of wheat in the Great Plains, which results in tissue necrosis and leads to inevitable losses to grain yield and quality attributes. The significance of this region suggests it could be exploited as a diagnostic marker for rapid identification of the allele present at this particular locus.
Finally, a total of 112 SNPs (S1B_4206166- S1B_4342143, S1B_6194001- S1B_141152195 and S1B_17068160-S1B_18683593) showed significance for grain yield across this region with 86 SNPs directly mapping to genes, including several stress responsive annotations. Most notable is the pentatricopeptide repeat-containing (PPR) protein (TraesCS1B01G039100), a stress responsive transcription factor (Figure 9). Research shows this region proved significant in 2014 and 2015, but not 2016. Additionally, a PPR protein has been shown in Arabidopsis to result in abiotic stress tolerance when overexpressed, suggesting a potential effective target of molecular manipulation for enhancement of crop productivity under stressful environments.
Genomic selection accuracy for quality traits
Considering the practicality of applying genomic selection, or GS, to the WIT VDP, evaluation factors that impact the effectiveness of adopting GS will be continued. In particular, the impact of environmental factors on GS performance, and thus the accuracy of GS, was assessed by training the Buster population in one season and then predicting line performance in another based upon one of several algorithms. This cross-year validation resulted in two groups: forward prediction (Figure 10A) and backward prediction (Figure 10B). Forward prediction represents scenarios where a previous year is used as a training population (TP) to predict performance in a subsequent year. For prediction of year 2015 field performance, GS was applied using 2014 to train or develop the model; this scenario was denoted as 20142015 in Figure 10A. Backward prediction simply is the reverse. Although forward predictions are consistent with practice, backward predictions were examined to more thoroughly understand GS applicability across more environment combinations.
As shown in Figure 10, gluten quality measured by adjusted SDS sedimentation volume, as well as wheat kernel hardness (NIR or SKCS) provided the most stable prediction outcomes across growing seasons. Overall, these three quality parameters achieved more than 50 percent prediction accuracy, compared to 34 percent prediction accuracy for grain yield averaged across environments (Figure 10B). Oddly and disappointingly, flour yield and mixograph performance exhibited poor predictability in general. For instance, predictability for key traits such as peak mixing time and mixing tolerance score produced a mean of 20 percent accuracy. Also, based on results, a key quantitative descriptor of the mixogram, mixograph tail width, had essentially no predictability (Figure 10). Thus, GS for mixograph tail width is not advisable.
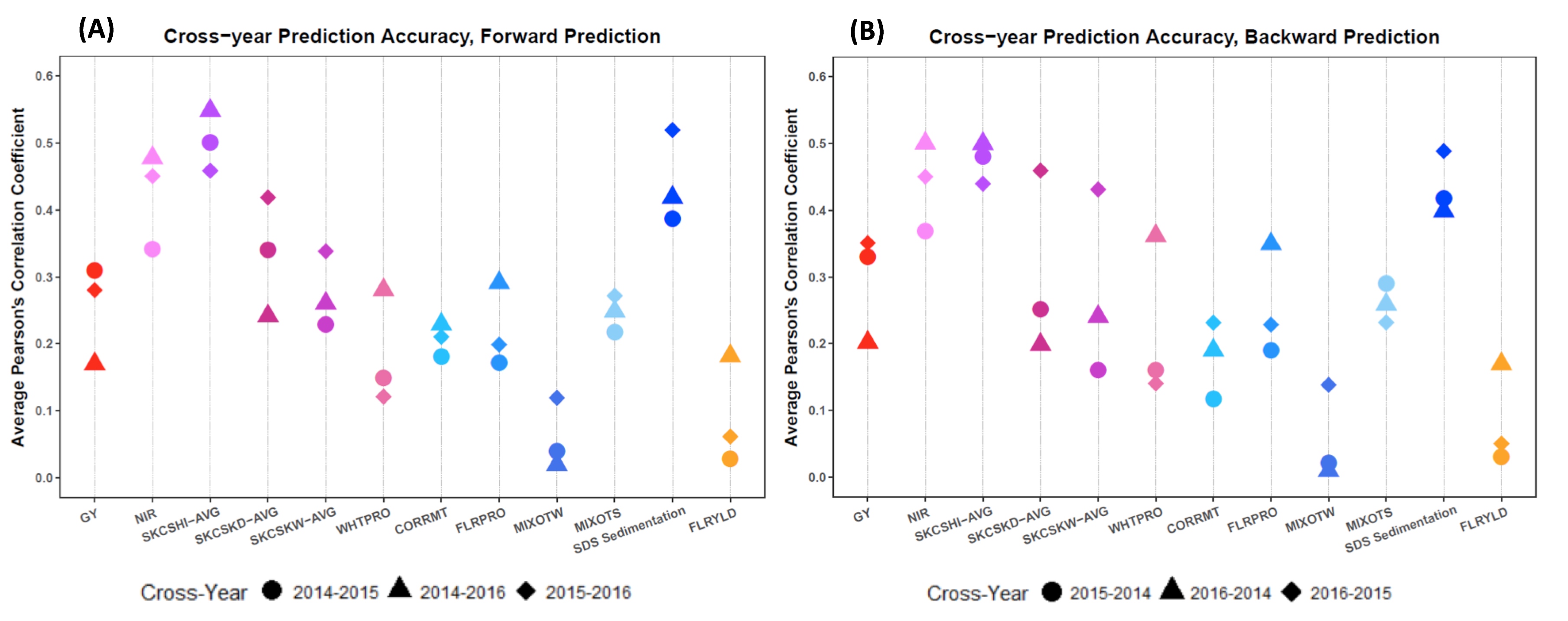
GY: grain yield
NIR: near infrared reflectance kernel hardness
SKCSHI-AVG: singe kernel characterization system kernel hardness, average value of 300 kernels
SKCSKD-AVG: singe kernel characterization system kernel diameter, average value of 300 kernels
SKCSKW-AVG: singe kernel characterization system kernel weight, average value of 300 kernels
WHTPRO: wheat protein content adjusted to 12% moisture
CORRMT: corrected mix time
FLRPRO: flour protein content adjusted to 14% moisture
MIXOTW: mixograph tail width at 2 minutes past peak dough development
MIXOTS: mixograph tolerance score on a 0-to-6 scale
SDS Sedimentation: adjusted sodium dodecyl sulphate sedimentation volume
FLRYLD: corrected flour yield adjusted to 14% moisture
Figure 10. Across-year genomic selection, or GS, prediction for grain yield and end-use quality traits. A) Averaged GS accuracy for forward prediction, i.e., GS models trained in one growing season and phenotypic values predicted for the following growing season. B) Averaged GS accuracy for backward prediction. Backward predictions were performed for the purpose of examining GS capacity across more environmental conditions. Prediction accuracies were averaged from all GS algorithms.
Genotype-environment interaction modeling
Since genetic and environmental variability influence grain composition and end-use characteristics, reliable line selection based on GS will depend on how GS models account for genotype-environment (GE) interaction. To achieve this research objective, WIT proposes a novel GS methodology capable of capturing variation across growing seasons. This new GS model expands upon the conventional use of linear models and provides the capacity of simultaneously modeling genetic effects of SNP predictors and GE interaction. Also, to accurately predict overall line performance across environments, a new cross-validation (CV2) procedure was proposed to examine the capacity of this new GS algorithm. The difference between CV1 and CV2 is shown in Figure 11. By phenotyping a different subset of individuals for each environment in CV2, rather than the same subset for all environments in CV1, CV2 further allows correlation of phenotypic values from both within- and across-environments to be modeled, with the same phenotyping cost.
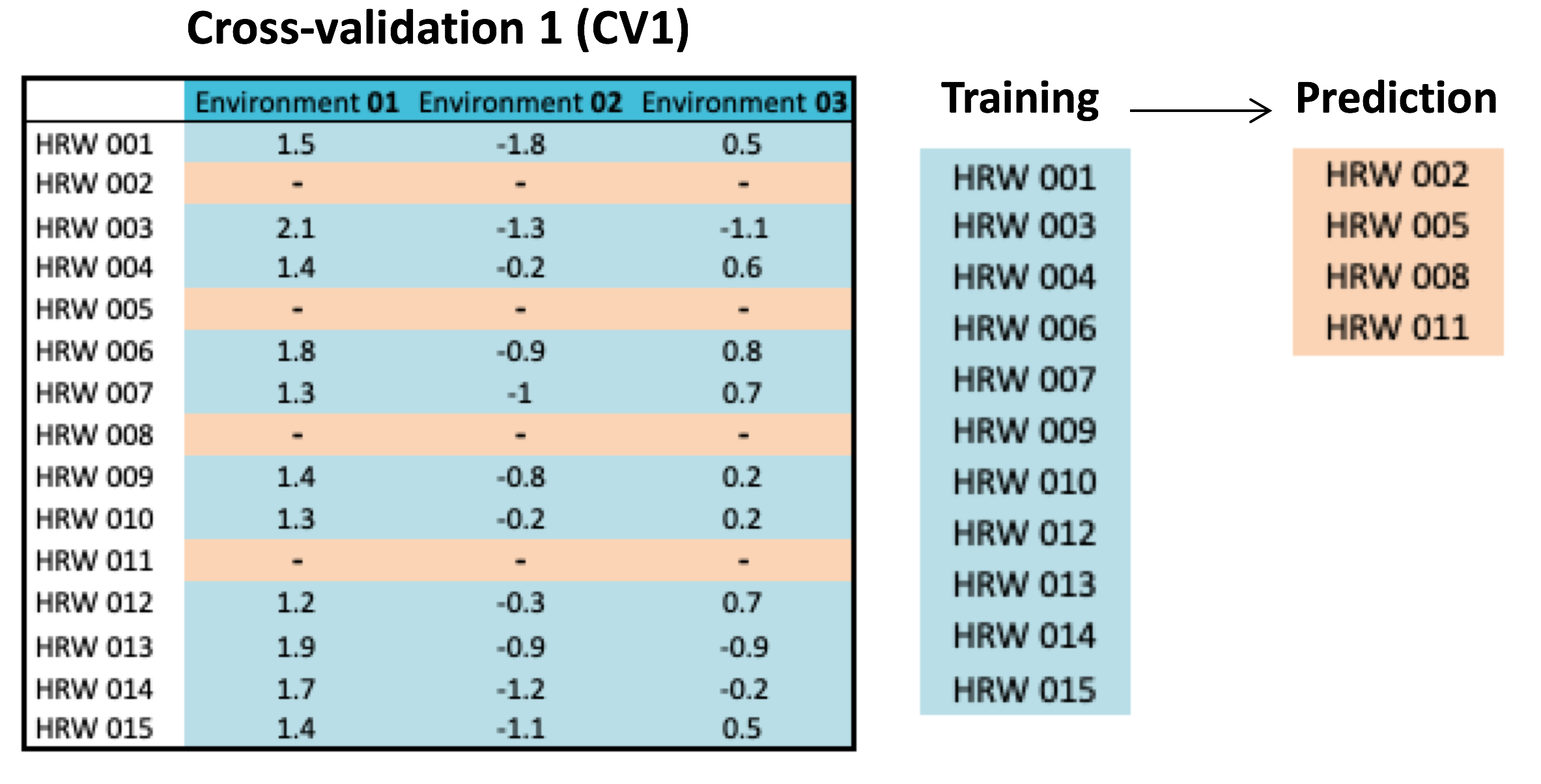
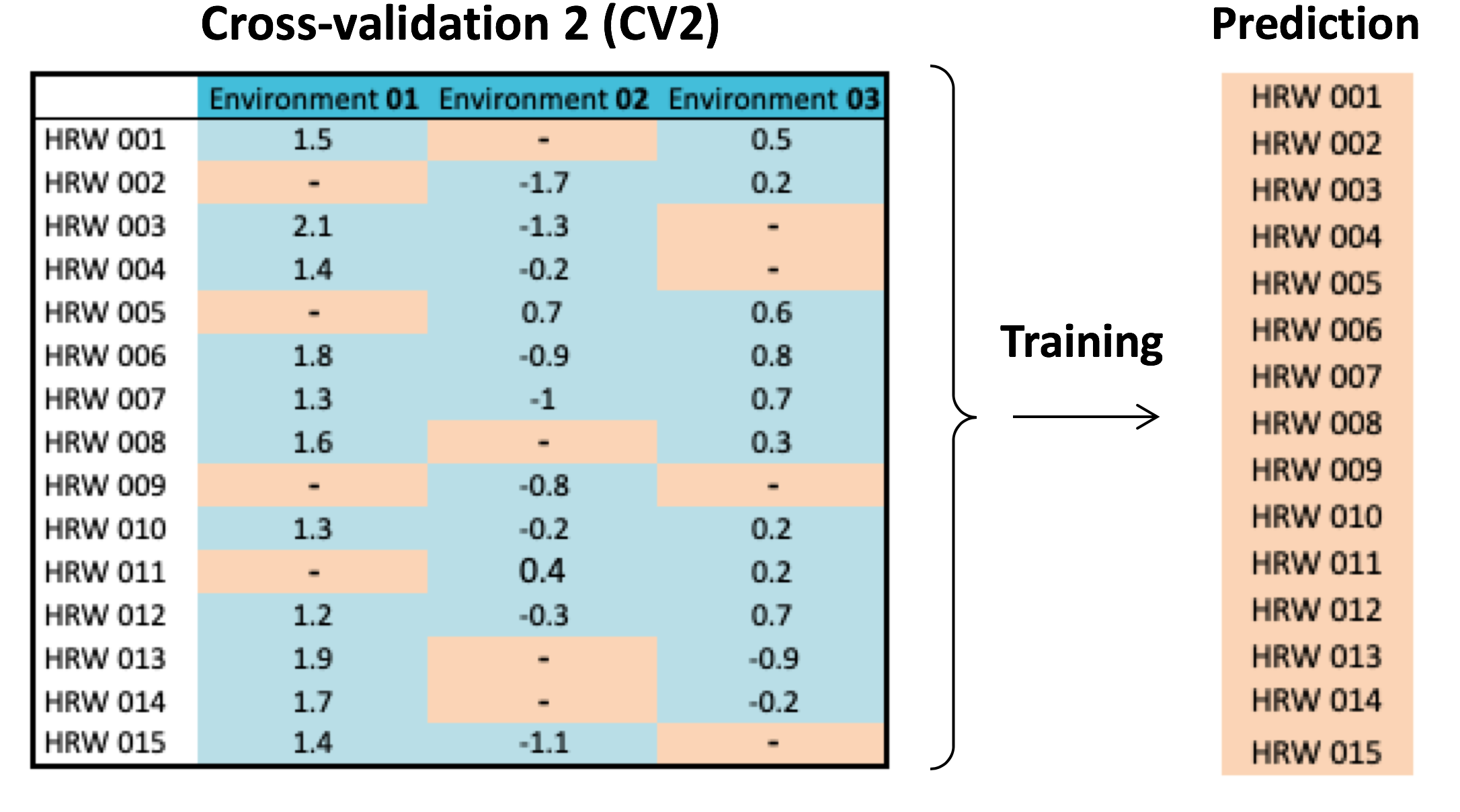
Figure 11. Illustration of genomic selection cross-validation (CV) schemes, CV1 versus CV2. While phenotyping costs in CV1 and CV2 are the same, CV2 has the advantage to perform prediction by training conducted within and across environments.
Overall, the benefit of incorporating GE into the multivariate GS model is evident in Figure 12; in all comparisons, GS performance using single-environment prediction models is the worst-case scenario in all four traits examined. For example, prediction accuracy for grain yield was increased from 31 percent in forward prediction (Figure 10A) and 55 percent in a single-environment GS model (Figure 12) to 62 and 79 percent when predicting years 2015 and 2016, respectively, with the multivariate GS algorithm (Figure 12). Further, adjusted SDS sedimentation volume remains the most predictable end-use quality trait, reaching an accuracy of 78 percent when a multiple-environment model is considered with SNP effects modeled in the weighted kernel model (Figure 12).
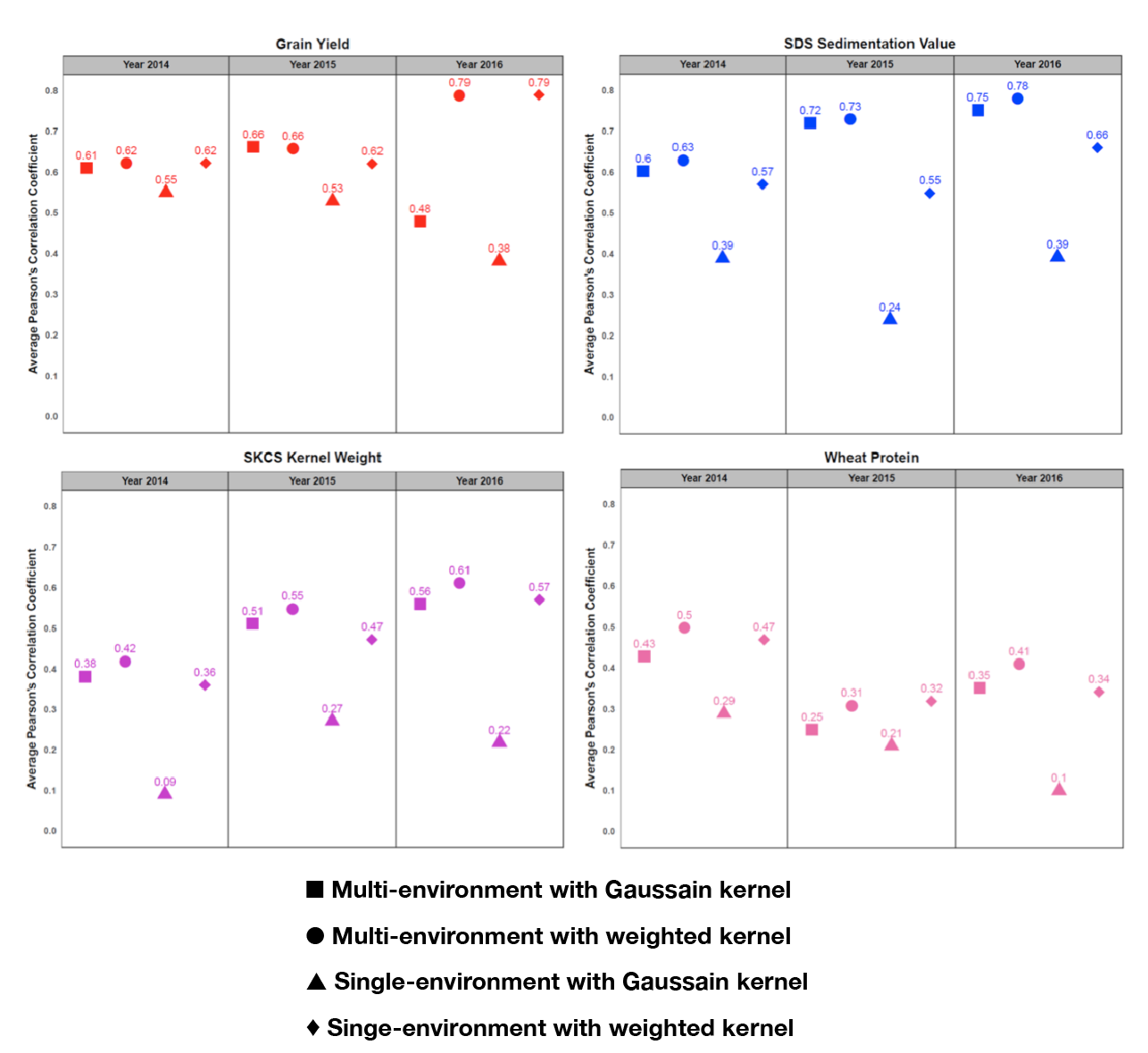
Figure 12. Prediction accuracy by average Pearson’s correlation coefficients from 50 replications of CV2 for multi-environment GS model using Gaussian kernel and with weighted kernel for grain yield and end-use quality characteristics. Results of single-environment GS model with Gaussian kernel and with weighted kernel are showed for comparison.
Nitrogen-use Efficiency at the Genetic Level
Brian Arnall
Plant and Soil Sciences
Experiments were conducted near Stillwater at the Lake Carl Blackwell Research Farm (LCB) and near Lahoma at the North Central Research Station (NCR). The study consisted of four cultivars (Gallagher, Smith’s Gold, Green Hammer and Lonerider) at four rates of pre-plant nitrogen (40, 80, 120 and 150 pounds nitrogen per acre). These rates were higher than the year before, which was 30, 60, 90 and 120 pounds nitrogen per acre, because no yield plateau was identified at LCB. All varieties were planted at a seeding rate of 67 pounds of seed per acre. At LCB and NCR, the plots were no-tilled into standing wheat stubble. Stand establishment at both locations was superb. At both locations, a post-emergence herbicide application of zidua, axial and metribuzin was utilized as a broad spectrum weed management strategy. At no time was weed competition a problem for these locations. In addition to the herbicide, both locations were managed with a two-pass fungicide program. At jointing, Quilt® was applied with an insecticide, while Approach® was applied at flag leaf emergence. No disease was observed within the trials.
Grain yield from LCB showed a strong response to nitrogen fertilizer across all varieties, with most reaching maximum yield potential at 120 pounds nitrogen with a 20 bushels per acre difference between the lowest and highest nitrogen treatments (Figure 13). The increase in nitrogen rate allowed researchers to observe the varieties under excessive nitrogen. Also at LCB, a significant increase in protein was observed with increasing nitrogen rates across all varieties. The increase in nitrogen increased protein by 2 percentage units for most varieties. As was hypothesized, variety did impact protein level. Figure 14 and Table 7 demonstrate how Green Hammer produced greater wheat protein content than Gallagher, Smith’s Gold and Lonerider, when nitrogen was limited or near the optimum rate (40, 80 and 120 pounds of nitrogen). However Green Hammer did yield lower than Gallagher and Smith’s Gold.
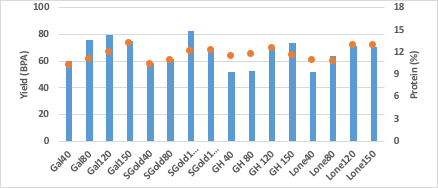
Figure 13. Grain yield (bushels per acre) and protein content (%) of four varieties Gallagher (Gal), Smith’s Gold (SGold), Green Hammer (GH) andLonerider (Lone) grown in four rates of nitrogen (40, 80, 120 and 150 pounds per acre) at the Lake Carl Blackwell Research farm.
Unfortunately, due to drought conditions at Lahoma, yields were well below expectation. Most varieties reached maximum potential at 80 pounds of nitrogen per acre at a yield range of 35 to 40 bushels per acres (see Figure 14). Much like at LCB, Green Hammer yielded slightly below the other cultivars, but had significantly higher protein content when nitrogen rate was below optimum (see Figure 14 and Table 7). At both LCB and Lahoma, the protein content of Green Hammer was 112 percent and 113 percent that of Gallagher at the 40-pound rate.
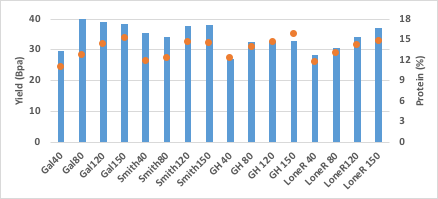
Figure 14. Grain yield (bushels per acre) and protein content (%) of four varieties Gallagher (Gal), Smith’s Gold (SGold), Green Hammer (GH) andLonerider (Lone) grown in four rates of nitrogen (40, 80, 120 and 150) at the Lahoma Research Station.
Table 7. Grain yield (bushels per acre) and protein content (%) of three varieties (Smith’s Gold, Green Hammer and Lonerider) grown in four rates of nitrogen (40, 80, 120 and 150 pounds per acre) compared against the local standard variety Gallagher. Data is reported from two locations Lake Carl Blackwell Research Farm and the Lahoma Research Station.
| Lake Carl Blackwell | Lake Carl Blackwell | Lake Carl Blackwell | |
|---|---|---|---|
| Variety | N Rate | Bushels | % Gal |
| Gallagher | 40 | 60 | |
| Gallagher | 80 | 76 | |
| Gallagher | 120 | 79 | |
| Gallagher | 150 | 75 | |
| Smith’s Gold | 40 | 59 | 98 |
| Smith’s Gold | 80 | 62 | 81 |
| Smith’s Gold | 120 | 83 | 104 |
| Smith’s Gold | 150 | 70 | 94 |
| GreenHammer | 40 | 52 | 86 |
| GreenHammer | 80 | 53 | 70 |
| GreenHammer | 120 | 69 | 87 |
| GreenHammer | 150 | 73 | 98 |
| Lonerider | 40 | 52 | 87 |
| Lonerider | 80 | 63 | 84 |
| Lonerider | 120 | 71 | 89 |
| Lonerider | 150 | 71 | 94 |
| Lake Carl Blackwell | Lake Carl Blackwell | |
|---|---|---|
| Variety | Protein | % Gal |
| Gallagher | 10.2 | |
| Gallagher | 11.1 | |
| Gallagher | 12 | |
| Gallagher | 13.2 | |
| Smith’s Gold | 10.3 | 101 |
| Smith’s Gold | 10.8 | 98 |
| Smith’s Gold | 12.1 | 101 |
| Smith’s Gold | 12.2 | 92 |
| GreenHammer | 11.4 | 112 |
| GreenHammer | 11.7 | 106 |
| GreenHammer | 12.4 | 104 |
| GreenHammer | 12.4 | 94 |
| Lonerider | 10.9 | 108 |
| Lonerider | 10.7 | 96 |
| Lonerider | 12.9 | 107 |
| Lonerider | 12.9 | 98 |
| Lahoma | Lahoma | |
|---|---|---|
| Variety | Bushels | % Gal |
| Gallagher | 30 | |
| Gallagher | 40 | |
| Gallagher | 39 | |
| Gallagher | 38 | |
| Smith’s Gold | 35 | 119 |
| Smith’s Gold | 34 | 86 |
| Smith’s Gold | 38 | 97 |
| Smith’s Gold | 38 | 99 |
| GreenHammer | 27 | 90 |
| GreenHammer | 33 | 82 |
| GreenHammer | 33 | 84 |
| GreenHammer | 33 | 86 |
| Lonerider | 28 | 95 |
| Lonerider | 31 | 76 |
| Lonerider | 34 | 88 |
| Lonerider | 37 | 96 |
| Lahoma | Lahoma | |
|---|---|---|
| Variety | Protein | % Gal |
| Gallagher | 10.9 | |
| Gallagher | 12.8 | |
| Gallagher | 14.4 | |
| Gallagher | 15.2 | |
| Smith’s Gold | 11.8 | 108 |
| Smith’s Gold | 12.3 | 96 |
| Smith’s Gold | 14.6 | 102 |
| Smith’s Gold | 14.6 | 96 |
| GreenHammer | 12.3 | 113 |
| GreenHammer | 14 | 109 |
| GreenHammer | 14.6 | 102 |
| GreenHammer | 15.8 | 104 |
| Lonerider | 11.7 | 107 |
| Lonerider | 13 | 102 |
| Lonerider | 14.2 | 99 |
| Lonerider | 14.9 | 98 |
In the 2017-18 crop year, Green Hammer was included in 13 variety performance trials. In 11 of the 13 trials, it ranked in the highest statistical grouping for wheat protein, with the next best being Doublestop, which was in the highest ranking in 12 of 24. The yield of Green Hammer was not in the highest grouping (only three of the 13 locations); however, it was always at or above the location mean.
In summary, the data from the second year of this project and the variety performance trials supported the results from 2017. The results from LCB and Lahoma aligned with those from Tipton to suggest a tendency for Green Hammer to maintain protein levels even at sub-optimum nitrogen levels. The ability of Green Hammer to reach yield levels at or just under that of Gallagher and Smith’s Gold was a positive outcome.
Moving forward, this study which focused on just a few lines will be discontinued in 2019. The nitrogen use efficiency work at Tipton, however, will be expanded. Instead of testing all lines under nitrogen stress and just a few lines under optimum nitrogen, lines will be tested with three rates of nitrogen (extreme stress 25 percent optimum, moderate stress 50 percent optimum and optimum nitrogen). Additionally in 2019, a full integrated pest management protocol will be implemented with a two-pass fungicide plan. Historically, Tipton was left untreated to observe resistant reactions. However, there is a strong probability that lines with good nitrogen use efficiency traits may have been lost due to poor pathogen resistance.
Wheat Breeding and Variety Development
Brett Carver
Plant and Soil Sciences
Just when foliar diseases were thought to be a common occurrence again in Oklahoma wheat production, the 2017-18 crop year proved that expectation wrong. Other than a brief appearance of powdery mildew and stripe rust at Chickasha, foliar diseases had essentially no impact on final yields in wheat breeding nurseries scattered across the state. What did have tremendous impact, either directly or indirectly, were the multiple freeze events in April, as described in more detail in the final chapter of this report entitled, “Wheat Variety Trials,” Page 44.
The direct effect could be observed by early May at Lahoma in the form of aborted tillers and reduced spike frequency (Figure 15). Though not observable until harvest, the indirect effect was in the form of smaller kernels, seemingly caused by delayed flowering following the spring freeze events, combined with a normal to accelerated physiological maturity pattern. The net result was a compressed kernel-filling period to which this breeding program had little exposure since spring 2012. Combined with season-long drought stress and the lack of disease pressure in the field, the environmental conditions of the 2017-2018 crop season left yet another indelible and unique imprint on genetic makeup of the OSU wheat variety development pipeline.

Figure 15. Contrasting reactions to April freeze events at Lahoma on May 4, 2018, representing 0 percent spike loss (left) and near 100 percent spike loss (right) for two advanced lines. Neither line had a prior history of freeze susceptibility in statewide trials.
The other consequence of these conditions (drought, freezes, lack of disease) was high yield compression in effectively every one of the 70+ multi-site breeding nurseries where grain yield differences normally provide an essential filter to select high-performing lines. Sometimes yield compression occurs as a simple consequence of reduced genetic divergence. In other words, genetically similar material will often produce similar results. That was not the case in 2018 when, even among the lines with wide divergence, the difference between top performer and bottom performer was less than 10 percent of the mean of the nursery. The 10 percent value is often a critical breakpoint for declaring yield differences as statistically significant and thus meaningful to the breeder.
Five or six to four
One visible impact on the variety development pipeline was a reduction in candidate varieties forwarded to OAES for release consideration. Entering spring 2018 and the wheat field tour season in May, WIT considered five or six experimental lines worthy of a release recommendation during summer 2018: OK12716, OK13209, OK13621, OK13625, OK12DP22004-016 and OK12206-127206-2. The last two have since been either dropped from further consideration (OK12DP22004-016) or postponed (OK12206-127206-2) for additional data collection in 2018-2019.
OK12206-127206-2 is currently WIT’s best HRW beardless line at this stage of advancement, but it has a history of wide variability in test weight from unacceptable to above average. OK12206-127206-2 exhibits an exceptional range of disease resistance in addition to Hessian fly resistance and very good end-use quality. This candidate will remain under evaluation while introducing another beardless candidate, OK11208, with potentially higher yielding ability and acceptable test weight or end-use quality. Three maturity types were identified in 2018 and have now been segregated into distinct but uniform lines for direct comparison with OK12206-127206-2 in 2018-2019.
As for the remaining four experimental lines, all were approved by OAES for release in late summer 2018. Four varieties may seem an excessively high number to launch in just one year. Their differences, however, in intended use or expected positioning justified such an unusual event.

Showdown (OK12716) features a relatively high yield ceiling in a grain-only production system and offers complete adaptation to a dual-purpose management system with good canopy closure at adequate seeding density, outstanding forage regeneration and grazing recovery (related to its more prostrate growth habit) and Hessian fly resistance. It will be marketed under the GrazenGrain® brand. Test weight is in the Endurance range and certainly not as high as Doublestop CL+. Disease resistance is broad and strong with the possible exception of leaf rust when present before heading at the level observed in 2017. Adaptation is very wide, extending from the Rolling Plains of Texas to central Kansas, including the Oklahoma panhandle. This adaptation zone almost combines the two adaptation zones of Bentley and Lonerider, though Lonerider may out-yield Showdown in some far western environments. Its parentage includes an OK Bullet sister and an AgriPro experimental line.

Green Hammer (OK13209) offers a critical yield protection advantage that could call for lower input costs. It carries a highly effective level of dual resistance to leaf rust and stripe rust, thus often neutralizing the positive effect of a fungicide application based on trials in Oklahoma and Kansas. Protein content has averaged about 1 percentage point higher than Gallagher, at a similar test weight level. Altogether, Green Hammer is considered OSU’s best offering at this time for combining disease and Hessian fly resistance, protein content, protein quality, and test weight into one variety. Its region of adaptation is centered on southwest, central and north-central Oklahoma. Green Hammer is a progeny of the three-way cross of OK Bullet/TAM 303 sister//Shocker. Note that TAM 303 was one of the two parents of Bentley. Green Hammer will be marketed under the GrazenGrain® brand.

Baker’s Ann (OK13621) will be licensed as a premium-quality wheat variety well suited for quality-based contracted production at a yield potential comparable to Green Hammer and Showdown. Baker’s Ann produces smaller seed than Gallagher (similar to Iba) at about 0.5 percentage point higher wheat protein, and qualitatively stronger dough to the degree that the Wheat Quality Council has classified this variety as a good blending wheat to correct for poor strength elsewhere. Baker’s Ann exhibits exceptionally strong resistance to stripe rust across a wide geography, though resistance to leaf rust may need to be bolstered with a fungicide application. It will fit best in the Oklahoma panhandle and north-central Oklahoma, and originates from the cross TAM 303 sister/Billings. Owing to its TAM 303 relationship, Baker’s Ann will carry the brand of GrazenGrain® but will carve a greater reputation under the GoldnGrain™ banner of premium quality.

Skydance (OK13625) also will be licensed as a GoldnGrain™ premium-quality wheat variety for dual functionality in bread and tortilla manufacturing. Its kernel size is similar to Gallagher, and test weight is at least one pound higher than Gallagher. Protein levels exceed Gallagher by about 0.5 percentage point. Skydance also has been found to tolerate suboptimal nitrogen availability and appears to be suited for certified organic production in its normal area of adaptation, which includes southwest and central Oklahoma, but extending, albeit at greater risk, into northern Oklahoma. Its disease package is outstanding, lacking only in BYD resistance (moderately susceptible). Skydance’s parentage includes a Fannin sister and Billings.
In summary, Showdown will cater to the commodity wheat market, whereas Baker’s Ann and Skydance are intended to service the value-capture wheat market that places a premium on improved functionality in general and dough strength in particular. Green Hammer will likewise do the same if managed accordingly, but it carries the additional ability to attract investors of elevated protein content. Expected adaptation zones are provided in Figure 16.
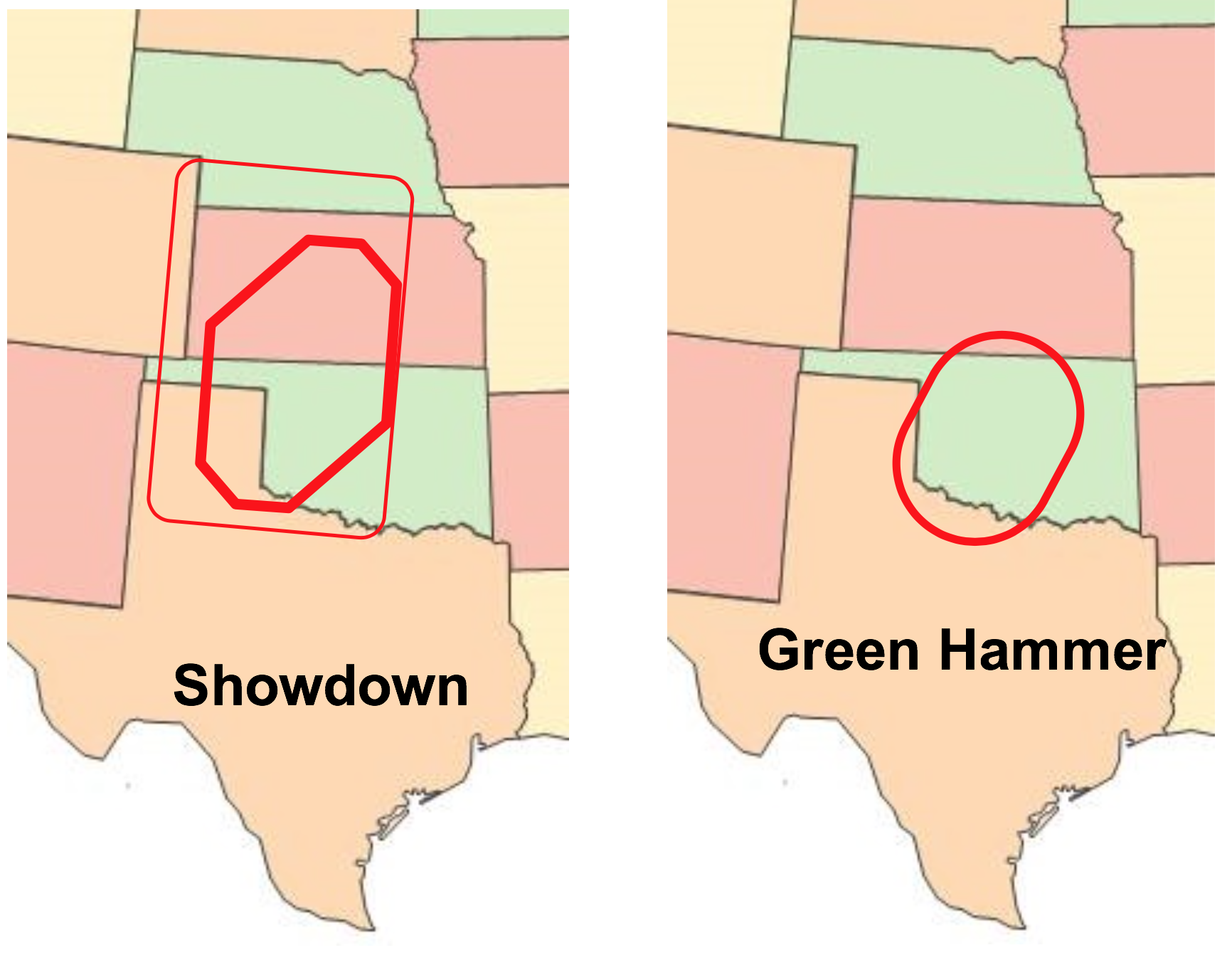
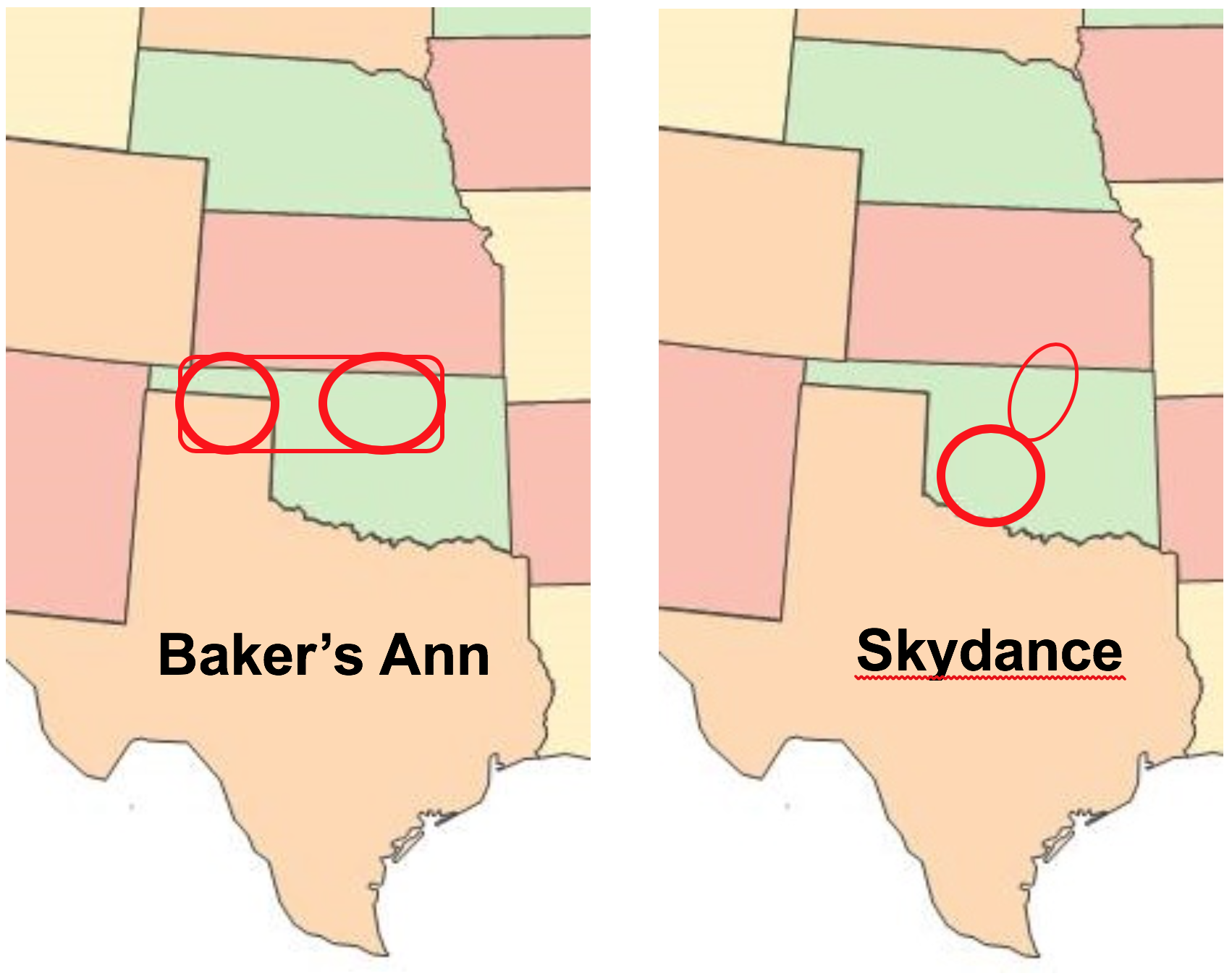
Figure 16. Adaptation maps for the four newest wheat variety releases by OAES in 2018. Zones indicated by shapes with greater weight represent primary areas of intended positioning.
BYD resistance now in the pipeline
Among the four newest wheat variety releases, one trait deficiency in common is a desirable level of BYD protection. The primary reason for this weakness is genetics; that is, none of the four varieties claim Duster as a parent, or even as a grandparent, and Duster is WIT’s most common source of BYD tolerance in the OSU VDP. It is certainly not the only one when Garrison comes to mind, but Garrison has contributed little to the germplasm downstream in the pipeline due to its acute susceptibility to races of stripe rust that emerged in spring 2012 in the Great Plains. Duster derivatives with better BYD protection than most would be Gallagher, Iba and Smith’s Gold (but not Lonerider). However, even the level of resistance present in those varieties is not perfect or complete.
What would make their BYD resistance more complete would be the addition of a unique gene source, that when stacked with the Bdv1-conferred resistance from Duster produces an additively higher level of resistance. The second gene for BYD resistance targeted by WIT for almost 10 years is called Bdv3, transferred from soft red winter wheat germplasm developed by Purdue University. WIT has come to informally call this two-gene stack BYD2G, now present in multiple lines entering release candidacy in 2018-2019 (Table 8). Of the experimental lines listed in Table 1, OK16D101089 and OK16D101073 were placed on first-year seed increase with OFSS in fall 2018. More desirable baking quality makes OK16D101089 the early favorite, though OK16D101073 may have slightly higher yield potential. Head-to-head comparisons of OK16D1010089 versus Gallagher in 2018 replicated yield trials (in the absence of BYD) produced yield differentials of +7, +7 and -11 bushels per acre at Lahoma, Chickasha and Okmulgee, respectively. A differential of 7 or more bushels per acre typically signified statistical significance in 2018.
Resistance to BYD was visibly obvious in harvest years 2017 and 2018 for all lines listed in Table 8. Striking examples of this newfound resistance level are provided in Figures 17 and 18 from photographs taken at Stillwater, where BYD severity levels are routinely higher than any other site WIT breeder trials might be conducted.
Table 8. Advanced WIT experimental lines selected from 2017-2018 breeding trials featuring very desirable to exceptional levels of barley yellow dwarf (BYD) resistance due to the presence of gene Bdv1 (from Duster usually), Bdv3 from Agropyron intermedium, and/or possibly other minor but unknown sources. For comparison, the BYD protection level of Duster is typically rated 2 or 3, depending on the level of disease pressure. Experimental lines highlighted in boldface are given highest priority entering into the 2018-19 crop season.
| Line | Recipient parent | No. of known BYD-R genes | BYDa | HFa |
|---|---|---|---|---|
| OK16D101089 | Bentley | 2 | 1 | 5 |
| OK16D101073 | Bentley | 1 | 1 | 5 |
| OK16107202 | OK10315 | 1 | 1 | 2 |
| OK14P212 | Duster | 1 | 2 | 1 |
| OK16D101072 | Bentley | 2 | 1 | 5 |
| OK16D101105 | Bentley | 1 | 1 | 1 |
| OK16D101113 | Bentley | 2 | 1 | 1 |
| OK16DIB127 | Bentley | 1 | 1 | 5 |
| OK16DIB128 | Bentley | 1 | 1 | 5 |
| OK16107125 | Doublestop CL+ | 1 | 2 | 1 |
| OK16107131 | Doublestop CL+ | 1 | 2 | 1 |
| OK16107143 | Smith’s Gold | 2 | 1 | 1 |
| OK16107155 | Smith’s Gold | 2 | 1 | 1 |
| OK16107157 | Smith’s Gold | 2 | 1 | 1 |
| Line | Recipient parent | LRa | YRa | BQa |
|---|---|---|---|---|
| OK16D101089 | Bentley | 1 | 1 | 2 |
| OK16D101073 | Bentley | 1 | 1 | 4 |
| OK16107202 | OK10315 | 1 | -- | 2 |
| OK14P212 | Duster | 1 | 1 | 2 |
| OK16D101072 | Bentley | 3 | 3 | 5 |
| OK16D101105 | Bentley | 3 | 1 | 3 |
| OK16D101113 | Bentley | 2 | 2 | 3 |
| OK16DIB127 | Bentley | 1 | 1 | 3 |
| OK16DIB128 | Bentley | 2 | 1 | 3 |
| OK16107125 | Doublestop CL+ | 1 | -- | 1 |
| OK16107131 | Doublestop CL+ | 1 | -- | 1 |
| OK16107143 | Smith’s Gold | 3 | -- | -- |
| OK16107155 | Smith’s Gold | 2 | -- | 2 |
| OK16107157 | Smith’s Gold | 1 | 2 | 2 |
a Trait categories abbreviated as BYD, barley yellow dwarf; HF, Hessian fly; LR, leaf rust; YR, stripe rust; and BQ, baking quality. Values ≤2 are considered very desirable; those ≥4 are undesirable. No value (--) indicates inconsistent or insufficient data for postulation.
WIT’s goal is to follow up that kind of success with discovery and deployment of bird cherry-oat aphid (BCOA) resistance under the leadership of Xu, Page 13; and Giles and Zarrabi, Page 16. Barley yellow dwarf virus is transmitted by many aphids, but BCOA is the predominant one in Oklahoma. Previously, aphid colonies were developed and maintained, and a reliable and repeatable screening assay was developed. In 2017, selection pressure was applied upstream in the variety development pipeline to better ensure discovery and retention of resistant germplasm at more advanced stages of line testing. The first cycle of fixed breeding lines underwent observation and preliminary yield testing in 2018 following just one generation of seed increase. Among 416 lines evaluated in 2018, 133 were advanced for more intense yield and quality testing in 2018-2019. The goal at that point will be to identify about 30 lines with broad adaptation and BYD tolerance based on BYD field response and growth-chamber assays designed to validate earlier predictions of tolerance.
This research constitutes a major breakthrough in addressing a persistent direct (by plant injury) or indirect (by BYD transmission) hazard to wheat production in Oklahoma. The ultimate goal is to combine BCOA resistance with BYD resistance to produce the consummate wheat variety for use in Oklahoma’s wheat grazing systems.
Breakthrough in WSM resistance
Incorporation of WSM resistance into the variety development pipeline reached new and significant heights in 2018. A shortage of agronomically relevant candidates with WSM protection is no longer the real hurdle but rather acceptable quality or over-reliance on resistance to the vector alone, or the wheat curl mite.
Following sufficient foundation seed production in 2018, a commercial-ready candidate (OK168512) was submitted for testing in the 2018-2019 OSU Wheat Variety Trials. This line offers WSM protection via Wsm1, the same gene that confers an equivalent level of WSM resistance in Mace, based upon side-by-side field comparisons by USDA-ARS collaborators in Lincoln, Nebraska (Bob Graybosch and Gary Hein). As a precautionary measure, one additional experimental line (OK168513) has also progressed through one generation of foundation seed increase, but apparently lacks the dough strength of OK168512. Yet another experimental line, OK168517 will be featured in the 2018-2019 Oklahoma Elite Trial along with OK168512 and OK168513, just to ensure WIT has locked in on the right candidate for western Oklahoma. All three lines have shown strong and equal resistance to WSM when challenged with viruliferous mites in the field in Nebraska (Figures 2 and 19).

Figure 17. Barley yellow dwarf resistance of an experimental line containing only gene Bdv3, as observed on May 15, 2018, at Stillwater.


Figure 18. Barley yellow dwarf (BYD) resistance of an experimental line containing only gene Bdv3 (top) versus a susceptible line (bottom), as observed on May 15, 2018, at Stillwater. Note an unusually high level of green-leaf retention on the flag leaf and the penultimate leaf, unrelated to maturity in the line with BYD resistance.

Figure 19. Mite-transmitted virus screen conducted by USDA-ARS scientists at the USDA-ARS Agricultural Research and Development Center, Mead, Nebraska in 2018. Wheat streak mosaic-susceptible lines exhibit severe yellowing and stunting. Note resistance of Doublestop CL+ and OK168513. Photos provided by G. Hein and R. Graybosch.
Another experimental line, OK12612, which was featured in previous editions of this report, is no longer considered a candidate variety because it lacks the necessary level of uniformity, yield potential and bread-making quality. The emergence of the three lines mentioned above, and others upstream in the variety development pipeline, made this decision easier than it was just two years ago. WIT’s ultimate goal remains to combine Wsm1 (or Wsm2) with Cmc4 (curl mite resistance) in the same genetic background with minimal impact of yield-reducing genes linked to Wsm1.
Resistance to WSM also occurs in hard winter wheat without connection to a known causal gene, even within WIT’s own germplasm. WIT reported in the 2017 Partners in Progress Wheat Research Report that an entire nursery of 2,100 early-generation populations was decimated by curl mite infestation and subsequent WSM damage at Marshall. Surviving most of the damage were multiple check plots occupied by Joe and Doublestop CL+. WIT confirmed the putative resistance of Doublestop CL+ in 2018 using the same field screen mentioned above (Figure 19).
Breakthrough in stripe rust resistance
Other than a brief appearance in breeding trials at Chickasha in 2018, stripe rust pressure has been non-existent since spring 2016, amounting to two generations of selection in the absence of this key disease. Thus, selection pressure for stripe rust resistance in the breeding program would have been absent during the past two years, if not for field screening graciously offered and conducted by USDA-ARS scientists at Pullman, in cooperation with Washington State University. While the entire WIT pipeline has not been subjected to stripe rust, a significant part of the pipeline containing advanced experimental lines one to three years away from release was put to this test, and the results were highly encouraging in 2018.
Without belaboring the data, Table 9 shows only the two tails, or extremes, of the phenotypic distribution for stripe rust reaction among advanced WIT lines in Washington. Instead of reporting data for all 185 lines, only the best 12 and worst six lines are shown. The reaction was reported as a single, simplified composite score, which combines in numeric form the kind of reaction and the severity of reaction from two planting dates at each of two Washington field sites.
Table 9. Composite scores for stripe rust reaction of advanced WIT experimental lines generated from field screenings in Washington in 2018.
| Line | Composite score |
|---|---|
| OCW04S717T-6W | 20 |
| OCW03S580S-10-4,5F | 29 |
| OK16D101094 | 36 |
| Green Hammer | 39 |
| OK16729W | 41 |
| OK16727W | 44 |
| OK15DMASBx7 ARS 6-6 | 48 |
| OK16D101103 | 50 |
| Baker’s Ann | 53 |
| OK15DMASBx7 ARS 6-4 | 55 |
| OK16D101089 | 57 |
| OK14P212 | 60 |
| Lonerider | 351 |
| Duster | 356 |
| OK15DMASBx7 ARS 7-24 | 416 |
| OK15DMASBx7 ARS 7-17 | 435 |
| OK16117 | 441 |
| OK15DMASBx7 ARS 7-41 | 473 |
Based on this composite score, the hard white, beardless advanced line, OCW04S717T-6W, represented the highest level of resistance among all 185 lines. Also exhibiting extreme levels of resistance were a soft wheat experimental (OCW03S580S-10-4,5F), two hard white wheat experimentals OK16727W and OK16729W, two high gluten-strength experimentals that may be eventually positioned for contract-grain production OK15DMASBx7 ARS 6-4 and OK15DMASBx7 ARS 6-6, and two experimentals currently under foundation seed increase, OK16D101089 and OK14P212. Green Hammer and Baker’s Ann lived up to expectations for demonstrating high levels of stripe rust resistance.
This collaboration ensures constant selection pressure for stripe rust resistance in years when the disease is not present in Oklahoma. In Washington, pressure from the disease far exceeds what is observed commonly in Oklahoma; thus effective resistance in Washington nearly always carries over to effective resistance in Oklahoma. Most candidates forwarded in 2018 to Oklahoma Foundation Seed Stocks (OFSS) for foundation seed increase possess a strong level of resistance to current races of stripe rust in the western U.S.
WIT’s pipeline contains a four-way valve
While the kind and number of crosses performed each year establish a trunk for the entire variety development pipeline, a four-way valve in the pipeline creates multiple flow paths distinguishable by unique breeding tactics (Figure 20). The principle flow path remains through the GrazenGrain® breeding system.
Imagine a filter through which potentially millions of genetic combinations will pass through and a small proportion ultimately generates fixed genetic lines for eventual testing. WIT has employed such a specially designed filter — with the incorporation of grazing pressure at key points in the 10-year variety development process — to invoke selection for characteristics essential to both grain-only and dual-purpose management systems. From 1997 until 2017, this system was applied at the Wheat Pasture Center at Marshall for initial population development, and at Stillwater and Lahoma for subsequent line selection. Beginning in fall 2017, the grazing component of this breeding system was moved from Marshall to a more centrally positioned area near Okarche.
While the GrazenGrain® breeding system remains in place and continues to work very well, the filter used in this system may not be the best one for developing lines best adapted to the far western part of Oklahoma. The reason is that populations are developed (i.e., filtered) and subsequent lines are produced (thus genetically fixed) in central Oklahoma, not western Oklahoma. There are ways around this conundrum, such as stacking the genetic deck with parentage best adapted to western Oklahoma, but we now use the following more aggressive approach.
For about 10 percent of the 1,000+ crosses made per year, WIT is now growing the segregating bulk populations at Oklahoma Panhandle Research and Extension Center (OPREC) under near-dryland conditions, where irrigation is provided only if needed to perpetuate the crop. Independent subsets of the same populations are also cycled through the GrazenGrain® breeding system. This process can be considered running a smaller but highly targeted breeding program inside a larger and expansive breeding program. Progeny extracted from populations grown and selected at OPREC are more likely to be specifically adapted to High Plains climatic and edaphic conditions than those extracted from populations grown at Marshall or Okarche.
Figure 20. The OSU wheat variety development pipeline is, in practice, four pipelinesofgermplasm, each originating early in the breeding process, or one to three years after the cross.
WIT finished its fifth year of population development in 2018, and for only the second time in OSU history, wheat headrow families were successfully evaluated at OPREC in 2018 to begin the line development process (Figure 21). The primary motivation is to move the impact of the OSU wheat breeding program further west without physically having to move the program. Elite lines, which trace back to the first selected headrow families in 2017 should reach the variety trial stage by fall 2020.

Figure 21. Single 3-foot rows,calledheadrows because each originated from a single head in the previous generation, are shown June 7, 2018, at OPREC. Each row represents a genetically unique experimental line and forerunner to a released variety. The 2018 harvest marked completion of the second cycle of population advancement and line development strictly in the Oklahoma panhandle.
With increasing regularity, WIT is bypassing both the GrazenGrain® and High Plains paths in favor of doubled haploids, a breeding tool that creates inbred experimental lines within two years of executing the cross, using a specialized mating system and tissue culture. The production of doubled haploids has its disadvantages and limitations; thus only about 1 percent of the available crosses fit this tool. However, turning the 10-year breeding cycle over at a faster rate improves the potential rate of gain on a per-annum basis and puts a wheat improvement program on par with other breeding programs (for example, maize) where the cycle time is greatly shortened. Twelve percent of the experimental lines subjected to replicated yield and quality testing in 2018 were doubled haploids. This number has increased slowly since 2012 and will continue to do so during the next five years, perhaps to as high as 25 percent. Two released OSU varieties were developed as doubled haploids, Smith’s Gold and Lonerider. It is this same technology that will enable OSU to introduce the AXigenTM trait, as part of the CoAXiumTM wheat production system, in less than five to six years.
The fourth leg of the variety development pipeline is populated with experimental lines intensively selected for yielding ability using a modified pedigree-selection breeding method. Rather than advancing populations of plants through the early generations of the GrazenGrain® breeding system, desirable families are selected strictly for grain yield-determining characteristics, then selected again within those families the following generation. This repetitive process may continue until highly inbred lines are selected with the highest yield potential, prior to replicated yield and quality testing. In 2017 and 2018, WIT reached a turning point in this selection process to advance several thousand experimental lines for seed increase and subsequent testing. Up to about 10 percent of the total pipeline is expected to originate via this path.
In summary, this four-way valve adds breadth to the OSU wheat breeding program to address multiple needs of wheat producers from the panhandle to Green Country, while maintaining depth or sheer numbers of breeding lines from which to choose candidate varieties. Common to all branches of this pipeline is an emphasis on end-use or functional quality demanded by a complex milling and baking industry.
By the numbers
The various moving parts of a plant breeding program, including this one, can be likened to a musical canon in which essentially the same music is being played or sung starting at different times. Likewise, the same fundamental breeding procedure, or set of procedures, is followed starting with a new set of hybridizations each year. A freeze frame of the breeding program at any point in time reveals different parts of the process in motion, as enumerated and discussed further in Figure 22.
2,058
 The number of segregating populations cycled through the Grazen Grain® breeding system at Okarche and Lahoma in 2018. The Altus location was a complete
bust. About 30 percent of these populations were sufficiently inbred to allow extraction
of experimental lines for eventual testing and selection. One segregating population
usually generates 96 experimental lines.
The number of segregating populations cycled through the Grazen Grain® breeding system at Okarche and Lahoma in 2018. The Altus location was a complete
bust. About 30 percent of these populations were sufficiently inbred to allow extraction
of experimental lines for eventual testing and selection. One segregating population
usually generates 96 experimental lines.
60,763
 The number of first generation F5 experimental lines planted in 3-foot headrows. this number well exceeds out target
for the VDP (55,000). Unfortunately, selection pressure was light in 2018 due to the
absence of meaningful disease pressure. More that 2,500 of these lines were advanced
for observation in the conventional yield plots in 2019-2019.
The number of first generation F5 experimental lines planted in 3-foot headrows. this number well exceeds out target
for the VDP (55,000). Unfortunately, selection pressure was light in 2018 due to the
absence of meaningful disease pressure. More that 2,500 of these lines were advanced
for observation in the conventional yield plots in 2019-2019.
18,303
 The number of second-generation fixed lines dedicated to our centerpiece breeding
nursery in 2018, the Dual-purpose Observation Nursery, and key turning point for the
lines borne out of the GrazenGrain® breeding system. Only those progeny superior for grazing persistence and grain-only
yield potential are advanced for statewide yield testing. Eleven percent of these
lines were HW.
The number of second-generation fixed lines dedicated to our centerpiece breeding
nursery in 2018, the Dual-purpose Observation Nursery, and key turning point for the
lines borne out of the GrazenGrain® breeding system. Only those progeny superior for grazing persistence and grain-only
yield potential are advanced for statewide yield testing. Eleven percent of these
lines were HW.
1891
 Double haploid lines produced outside of, and which circumvent, the conventional GrazenGrain® system. This breeding system relies mostly on the elite parentage with high mean but
low genetic variant. This portion of the line-evaluation program is increasing each
yea, but the highest proportion is not likely to exceed 25 percent so that long-term
generic gains are not sacrificed for short-term gains.
Double haploid lines produced outside of, and which circumvent, the conventional GrazenGrain® system. This breeding system relies mostly on the elite parentage with high mean but
low genetic variant. This portion of the line-evaluation program is increasing each
yea, but the highest proportion is not likely to exceed 25 percent so that long-term
generic gains are not sacrificed for short-term gains.
9
 Out of 25 Great Plains varieties preferred by one of the largest wheat milling enterprises
in the U.S.,nine were developed by WIT: Ruby Lee, Billings, Duster, Doublestop CL+,
Bentley, Gallagher, Iba, Smith's Gold, and Spirit Rider.
Out of 25 Great Plains varieties preferred by one of the largest wheat milling enterprises
in the U.S.,nine were developed by WIT: Ruby Lee, Billings, Duster, Doublestop CL+,
Bentley, Gallagher, Iba, Smith's Gold, and Spirit Rider.
17
 Extraordinary number of candidate varieties advanced for seed increase by Oklahoma
Foundation Seen Stock in summer 2018.
Extraordinary number of candidate varieties advanced for seed increase by Oklahoma
Foundation Seen Stock in summer 2018.
1 and only 1
 Only one wheat team and one wheat variety development program. in the U.S. that focuses
on adaptation to the wheat/stocker cattle enterprise, without losing sight of what
steers wheat prices - quality. It does matter.
Only one wheat team and one wheat variety development program. in the U.S. that focuses
on adaptation to the wheat/stocker cattle enterprise, without losing sight of what
steers wheat prices - quality. It does matter.
Figure 22. The OSU wheat improvement program, by the numbers, for the 2017-2018 crop season.
Candidate variety lineup
Following the 2018 harvest and after thorough consideration of all advanced lines under breeder-seed increase in 2018, WIT submitted breeder seed of nine new HRW and HW candidates for grow-out and on-farm evaluation by OFSS in 2018-2019. Another eight candidates previously under Foundation Seed increase in 2018 were carried over to 2018-2019. Thirteen of these 17 candidates are characterized fully in Tables 10 and 11. If not for its Hessian fly susceptibility and average acid-soil tolerance, the one candidate that shines brightest by far has a white brancoat and lacks awns. Even with these two blemishes, OCW04S717T-6W may be the best candidate to explore a release option in 2019. The question becomes how best to launch a variety that the supply chain would stand to benefit immensely but would likely disrupt the same supply chain if not maintained, at least in part, in an identity-preserved production system. Further, threshability is not a characteristic easily determined with research-plot equipment. Indirect evidence in the form of test weight patterns provides WIT the best clue for threshability, and thus far, test weight of OCW04S717T-6W has been acceptable to above-average and several pounds heavier than the other beardless (but HRW) candidate under increase, OK12206-127206-2.
As already characterized above, the most likely candidates to fill the immediate release agenda in early or late 2019 are a BYD-resistant progeny of Bentley (OK16D101089); a WSM-resistant line for western Oklahoma if competitive ability holds up at least on par with non-WSM resistant lines such as Lonerider (OK168512); a new two-gene Clearfield line unique from Doublestop CL+ but with no drop-off in yieldability and geographic reach or quality (OK12912C-138407-2); and a hard white wheat highly competitive with Joe or Stardust in ways other than simply grain yield (OCW04S717T-6W).
Table 10. OSU candidate varieties placed under seed increase in fall 2018 with Oklahoma Foundation Seed Stocks. Number of years of Foundation Seed production indicated as of summer 2018. HRW elite, grey; WSM-resistant candidates, orange; Clearfield Plus candidates, dark orange; HW elite, no highlight.
| Candidatea | Pedigree | OFSS | Feature traits |
|---|---|---|---|
| OK14P212 | OK01307/Duster//OK06822W | 2 | See OK1059018 reseln, but less mildew resistance, greater test weight. |
| OK1059018 reseln | Billings/Duster | 1 | All-purpose: grazing, disease-resistant wheat with premium quality. |
| OK13P016 | Billings/Duster | 2 | Southern Oklahoma, dual-purpose intent; HF-R; high quality – bread/tortilla. |
| OK16D101089 | OK12621/Bentley | 1 | Significant breakthrough in BYD protection; unexplainable leaf rust res. |
| OK16D101073 | OK12621/Bentley | 1 | See OK16D101089, but lower quality/test weight and higher yield. |
| OK14124-2 | NI04430/OK05303//Fuller | 1 | High protein/dough strength/test weight. suitable for late planting? |
| OK12206-127206-2 | Y98-912/OK00611W//OK03716W | 3 | Beardless with disease & HF resistance; premium quality. |
| OK168512 | Overley+/Fuller//2*CSU exptl. | 2 | Moderate WSM resistance; better yielding ability of two sibs. |
| OK168513 | Overley+/Fuller//2*CSU exptl. | 2 | Highly WSM-resistant; moderate yield potential. |
| OK12912C-138407-2 | N91D2308-13/OK03926C//OK03928C | 3 | Doublestop upgrade for straw strength, forage production, maturity. |
| OK149132C | CO06054/ OK06029C | 1 | Beautiful & early; yield ceiling tops Doublestop; less disease protection. |
| OCW04S717T-6W | CIMMYT seln/KS exptl.//KS91W047 | 2 | Exceptional quality HW beardless; top-tier yield and disease protection. |
| OK14P736W | Australian sources/2*OK Bullet | 1 | Up and coming HW, but best suited for western Oklahoma. |
a Not listed here are three candidates: one featuring the soft red winter class adapted to eastern Oklahoma, Arkansas, Missouri and areas further north (OCW03S580S-8WF); a Gallagher reselection (reseln) with uniform stripe rust resistance and slightly later maturity (OK15818); and one beardless HRW candidate with statewide adaptation and from which three maturity classes were identified and recovered for separate increase.
Table 11. Trait categorya
Trait ratings (1-to-5 scale) for OSU candidate varieties placed under seed increase
in fall 2017 with Oklahoma Foundation Seed Stocks. Grey, HRW elite; orange, WSM-resistant
candidates; dark orange, Clearfield Plus candidates; no highlight, HW elite with pre-harvest
sprout tolerance.
| Candidate | DP | HF | YR | Weaknesses |
|---|---|---|---|---|
| OK14P212 | 1 | 1 | 1 | Mildew, shattering |
| OK1059018 reseln | 1 | 2 | 2 | Standability |
| OK13P016 | 1 | 1 | 1 | Mildew |
| OK16D101089 | 2 | 5 | 2 | Fine canopy texture |
| OK16D101073 | -- | 5 | 1 | Mix tol., yellow berry |
| OK14124-2 | 5 | 5 | 2 | Inopportune plant date |
| OK12206-127206-2 | 2 | 1 | 1 | Test weight |
| OK168512 | 2 | 2 | 2 | Kernel size |
| OK168513 | 2 | 2 | 2 | Kernel size |
| OK12912C-138407-2 | 1 | 3 | 1 | Tall |
| OK149132C | 1 | 5 | 2 | Yield protection |
| OCW04S717T-6W | 1 | 5 | 1 | April freeze |
| OK14P736W | 1 | 5 | 2 | WSBMnotWSSM |
| Candidate | LR | TS | Weaknesses |
|---|---|---|---|
| OK14P212 | 1 | 5 | Mildew, shattering |
| OK1059018 reseln | 1 | 5 | Standability |
| OK13P016 | 2 | 3 | Mildew |
| OK16D101089 | 1 | 4 | Fine canopy texture |
| OK16D101073 | 1 | 3 | Mix tol., yellow berry |
| OK14124-2 | 1 | 2 | Inopportune plant date |
| OK12206-127206-2 | 3 | 3 | Test weight |
| OK168512 | 3 | 2 | Kernel size |
| OK168513 | -- | 3 | Kernel size |
| OK12912C-138407-2 | 1 | 3 | Tall |
| OK149132C | 5 | 5 | Yield protection |
| OCW04S717T-6W | 1 | 1 | April freeze |
| OK14P736W | 4 | 2 | WSBMnotWSSM |
| Candidate | PM | V | AST | Weaknesses |
|---|---|---|---|---|
| OK14P212 | 4 | 1 | 2 | Mildew, shattering |
| OK1059018 reseln | 1 | 1 | 1 | Standability |
| OK13P016 | 4 | 1 | 1 | Mildew |
| OK16D101089 | 1 | 1 | 1 | Fine canopy texture |
| OK16D101073 | 1 | 1 | 1 | Mix tol., yellow berry |
| OK14124-2 | 4 | 1 | 1 | Inopportune plant date |
| OK12206-127206-2 | 1 | 1 | 2 | Test weight |
| OK168512 | 2 | 1 | 3 | Kernel size |
| OK168513 | 1 | 1 | 3 | Kernel size |
| OK12912C-138407-2 | 1 | 1 | 1 | Tall |
| OK149132C | 1 | 1 | 4 | Yield protection |
| OCW04S717T-6W | 1 | 1 | 3 | April freeze |
| OK14P736W | 2 | 3 | 3 | WSBMnotWSSM |
| Candidate | SS | BQ | Weaknesses |
|---|---|---|---|
| OK14P212 | 2 | 2 | Mildew, shattering |
| OK1059018 reseln | 3 | 1 | Standability |
| OK13P016 | 2 | 1 | Mildew |
| OK16D101089 | 1 | 2 | Fine canopy texture |
| OK16D101073 | 1 | 4 | Mix tol., yellow berry |
| OK14124-2 | 1 | 1 | Inopportune plant date |
| OK12206-127206-2 | 1 | 1 | Test weight |
| OK168512 | 1 | 1 | Kernel size |
| OK168513 | 2 | 2 | Kernel size |
| OK12912C-138407-2 | 1 | 1 | Tall |
| OK149132C | 1 | 2 | Yield protection |
| OCW04S717T-6W | 1 | 1 | April freeze |
| OK14P736W | 1 | 2 | WSBMnotWSSM |
a Trait categories abbreviated as DP, dual-purpose capability (forage and grain combined); HF, Hessian fly; YR, stripe rust; LR, leaf rust; TS, tan spot; PM, powdery mildew; V, WSBM/WSSM complex; AST, acid-soil tolerance; SS, straw strength; and BQ, baking quality. Values ≤2 are considered very desirable; those ≥4 are undesirable. No value (--) indicates inconsistent or insufficient data for postulation.
Wheat Variety Trials
David Marburger, Robert Calhoun, Brett Carver,
Branden Watson and Christopher Gillespie
Plant and Soil Sciences
Bob Hunger
Entomology and Plant Pathology
At the time of writing this report, 2018 Oklahoma wheat production is estimated to be 52.0 million bushels, 47 percent less than the 2017 production (Table 12) and 62 percent less than the 2016 production.
The lower total grain production is the result of fewer wheat acres harvested across the state, primarily from abandonment due to drought or baled for hay, and the below-average yield. The 4.3 million planted acres was down only 4 percent compared to the previous year, but that was still 18 percent lower than the previous 10-year average. The number of harvested acres is estimated at 2.0 million, which is 31 percent less than in 2017 (Table 12), and the lowest number in the state since 1913. The statewide average yield is projected at 26 bushels per acre. This is 8 bushels per acre (24 percent) less than the 2017 state average and 3.6 bushels per acre (12 percent) less than the previous 10-year average.
Table 12. Oklahoma wheat production for 2017 and 2018 as estimated by USDA NASS, June 2018.
| 2017 | 2018 | |
|---|---|---|
| Harvested acres | 2.9 million | 2.0 million |
| Yield (bu/ac) | 34 | 26 |
| Total bushels | 98.6 million | 52.0 million |
The 2017-2018 wheat growing season was a fight from start to finish for many producers across the state. The growing season got an early start due to an unusual August for Oklahoma. Temperatures were below normal, and rainfall totals were above normal for the month. This prompted producers interested in targeting fall forage to begin planting at the end of August. Planting continued to move rapidly through the Labor Day weekend, and most of the wheat during this time was sown into adequate soil moisture and emerged rapidly. Those producers who waited until after Labor Day to plant saw more unfavorable conditions as temperatures rose, and available soil moisture quickly dried up. Wheat planted during this time was “dusted-in” and finally received precipitation toward the end of the month into the beginning of October to get the seed to germinate. Wheat planting intended for grain-only was stalled during the average timeframe of early to mid-October due to these precipitation events. Once the ground dried enough, most producers were able to quickly make up time and get the crop planted, but some needed until November to finish.
After mid-October, the rain quit falling for the remainder of the calendar year. Crop conditions during the early part of the season were average but quickly deteriorated as the season progressed. This also led to a disappointing fall forage production and grazing season for most producers. Those who planted during late August to early September and were able to protect the crop from fall armyworm achieved good stands and had some available pasture later in the fall. However, those who waited until after Labor Day or later to plant were not as fortunate. The later planting and lack of precipitation resulted in low total fall forage production or no available pasture at all.
Drought conditions and average to below average temperatures persisted throughout January into February. Even for the producers who had available fall pasture, the drought conditions limited the overall number of days of grazing.
Some precipitation finally fell in parts of the state during late February into early March. For many fields, this was the first precipitation received since planting. Below average temperatures were observed coming out of winter, and plants broke winter dormancy later than normal. Below average temperatures persisted, resulting in slow overall growth and development during this time. The first hollow stem growth stage was reached for many varieties during the second to third week of March, which was seven to 10 days later than normal. Unfortunately, the rain received during late February to early March was not quite enough to give any grazed wheat the boost it needed to recover well.
Overall growth and development continued at a slower than normal pace due to the second-coldest April on record. Three separate and widespread freeze events also occurred during the first week of April, resulting in significant injury in some areas. Most wheat headed during mid- to late April because of the cooler temperatures, with this being seven to 14 days behind normal. The prevailing thought was that this would translate into a later-than-normal harvest. However, the cold temperatures in April were followed by the warmest May on record. The warm temperatures and lack of rainfall advanced the crop quickly at this point, resulting in suboptimal conditions for the grain-fill period.
Most wheat was mature in southwestern Oklahoma by the end of May and in the central to northern parts by early June. Producers for the most part were not delayed by rainfall events, and with the dry weather during June, much of the wheat was harvested timely and quickly. Overall, harvest was almost complete in the state by late June.
Yields throughout Oklahoma varied depending on location but were below average overall. Part of this variability was due to overgrazing and whether an area caught or missed a rainfall event during early spring. Field averages of 15 to 30 bushels per acre were the norm across much of the state, but higher averages, even into the 50 to 60 bushels per acre range, were not uncommon in some areas that received timely rainfall. Test weights throughout harvest remained at or above 60 pounds per bushel for early-harvested fields and did not drop much below the upper 50s towards the end of harvest. Protein content also remained at or above acceptable levels.
Different insects were a concern at times during the growing season, but few were widespread or season-long outside of the fall armyworm. Unless treated, the fall armyworm devastated those producers who planted in late August into early September. Many fields had to be replanted, and some producers commented that this was the worst that they had ever observed. Unfortunately, some reports indicated the fall armyworm was still causing damage into early November. The dry weather experienced across the state through the winter provided ideal conditions for winter grain mites and brown wheat mites to thrive on wheat plants coming out of winter dormancy, and there were some reports of fields warranting control. Aphids were not really on the radar screen of most producers until mid-March, but this pest was still not the limiting factor as observed in other years. Despite the low aphid numbers, Barley Yellow Dwarf (BYD) was evident in some fields as flag leaves and heads started to emerge. While there was quite a bit of leaf purpling and yellowing associated with BYD, there was not much stunting observed, with stunting resulting from “hot spots” of aphid pressure with early-season transmission of the virus. Wheat Streak Mosaic (WSM), transmitted by the wheat curl mite, was an issue again for producers in southwestern Oklahoma, but the overall impact of WSM was not as much as the 2016-2017 crop season. Reasons for this were related to later planting and emergence of some wheat; additionally, fields that may have had WSM were abandoned due to the drought or cut and baled for hay before symptoms could be observed.
Diseases were at low levels overall during the season primarily due to the drought conditions. Parts of central to south central Oklahoma did experience low levels of powdery mildew, leaf rust, and stripe rust. In some cases, powdery mildew could be observed high in the canopy. For the remainder of the state, it was difficult to find foliar diseases, especially during stem elongation into the grain-fill period. One disease more prominent than in years past was Fusarium foot dry (dryland root rot). Signs and symptoms of this disease appeared suddenly during early May as hot temperatures returned and the crop progressed through grain-fill. However, symptoms of this disease can appear similar to symptoms of premature death caused by freeze, drought and other conditions. In parts of the northwest and panhandle regions, symptoms of dryland root rot may have been confused with symptoms caused by the drought and/or freeze, whereas in others (such as the wheat variety trial at Lahoma), damage caused by the April freeze events was expressed distinctly earlier. Because of the impact that leaf rust and stripe rust have had over the past several years, producers were ready to apply a foliar fungicide to susceptible varieties, but unfavorable conditions for disease development did not warrant an application in most cases. Variety trial results from Apache and Lahoma indicated that producers in these areas were justified in not spraying, as no evidence of a positive response to a fungicide application was found. However at Chickasha, where low to medium levels of leaf and stripe rust and medium to high levels of powdery mildew were present, the two fungicide applications implemented at this location contributed to protecting the yield potential for a number of varieties compared to the non-treated plots of those same varieties.
Testing methods
Seed was packaged and planted in the same condition as it was delivered from the respective seed companies. Most seed was treated with an insecticide plus a fungicide, but the formulation and rate of seed treatment used was not confirmed or reported in this document.
Conventional-till plots were eight rows wide with 6-inch row spacing and were sown with a Hege small-plot cone seeder. No-till plots were seven rows wide with 7.5-inch row spacing and were sown with a Great Plains no-till drill modified for cone-seeded, small-plot research. With the exception of dryland locations in the Panhandle, plots were planted 25 feet long and trimmed to 19 feet at harvest with the plot combine. Panhandle dryland locations were 35 feet long at planting and trimmed to 29 feet at harvest. Wheel tracks were included in the plot area for yield calculation, for a total plot width of 60 inches. Experimental design for all sites other than Apache and Lahoma was a randomized complete block with four replications. Apache and Lahoma were a split-plot arrangement of a randomized complete block with four replications where whole plots were fungicide treated or non-treated, and sub-plots were wheat variety.
Conventional-till plots received 50 pounds per bushel of 18-46-0 in-furrow at planting. No-till plots received 5 gallons per acre of 10-34-0 at planting. The Marshall dual-purpose (DP) trial, Union City, Walters, and forage trials were sown at 120 pounds per acre. The Panhandle irrigated and dryland locations were sown at 90 and 45 lb/ac, respectively. All other locations were sown at 60 pounds per acre. Grazing intensity, nitrogen fertilization, and insect and weed control decisions were made on a location-by-location basis and reflect standard management practices for the area.
Plots were harvested with a Hege or Winterstieger Delta small plot combine. Grain weight, test weight and moisture content were collected from each plot, and grain yields were corrected to 12 percent moisture content. Grain moisture at all sites was generally below 12 percent, and maximum and minimum grain moisture for all plots at a location typically ranged no more than 2 percent.
Data Interpretation
Yield and test weight data for each location and regional summary were analyzed using the appropriate statistical methods. At the bottom of each table, the mean and least significant difference (LSD) values are reported. The LSD is a test statistic that aids in determining whether there is a true difference in yield or test weight. In this report, one can be 95 percent confident that the difference between two varieties is real if the difference is equal to or greater than the LSD value. Data that is not significant is indicted by “NS.” For example, if the LSD value is four bushels per acre in a trial which Variety A yielded 30 bushels per acre and Variety B yielded 26, then Variety A would be considered to have a statistically higher yield. However, if Variety C yielded 27 bushels per acre, then Variety A and Variety C would be considered to have a similar yield. In that same example trial, there is a 5 percent chance that the four bushels per acre difference between Variety A and Variety B does not truly exist, but random chance caused the five-bushel difference. These chance factors may include differences in fertility, moisture availability and diseases for example. To aid in determining the varieties with the highest yields and test weights, values highlighted in gray do not differ statistically from the highest value within a column. The performance of a variety may vary from year to year, even at the same location. Tests over two or more years and over multiple locations more accurately predict variety performance.
Additional information online
A copy of this publication as well as additional information about wheat management can be found at:
Website: www.wheat.okstate.edu
Blog: www.osuwheat.com
Funding provided by:
Oklahoma Wheat Commission
Oklahoma Wheat Research Foundation
OSU Cooperative Extension Service
OSU Agricultural Experiment Station
Entry fees from participating seed companies
OSU Area Agronomists
Brian Pugh – Northeast District
Josh Bushong – Northwest District
Heath Sanders – Southwest District
Extension Educators
Thomas Puffinbarger, Alfalfa County
Loren Sizelove, Beaver County
David Nowlin, Caddo County
Kyle Worthington, Canadian County
Kimbreley Davis, Cotton County
Sug Farrington, Cimarron County
Ron Wright, Custer County
Rick Nelson, Garfield County
Shiann Burns, Grady County
Kassie Junghanns, Grant County
Darrell McBee, Harper County
Gary Strickland, Jackson and Greer counties
Zack Meyer, Kingfisher County
Troy Gosney, Major County
Courtney May, Ottawa County
Dr. Britt Hicks, Texas County
Greg Highfill, Woods County
Station Superintendents
Erich Wehrenberg, Agronomy Research Station, Stillwater
David Victor, North Central Research Station, Lahoma
Cameron Murley, Oklahoma Panhandle Research and Extension Center, Goodwell
Michael Pettijohn, South Central Research Station, Chickasha
Rocky Thacker, Southwest Research and Extension Center, Altus
Student Worker
Ankur Limaje
Summary 1: 2017-2018 Oklahoma Wheat Variety Performance Tests Summary
| Source | Variety | Afton | Altus | Alva | Apache |
|---|---|---|---|---|---|
| AGESCO | AG Gallant | - | - | - | - |
| AGESCO | AG Icon | - | 21 | - | |
| AgriMAXX | AM Eastwood | - | 9 | - | - |
| OGI | Bentley | 29 | 24 | 17 | 38 |
| AgriPro | Bob Dole | - | 7 | 16 | - |
| CROPLAN | CP78-26 | - | 19 | - | - |
| OGI | Doublestop CL Plus | 46 | 16 | 17 | 36 |
| OGI | Duster | 29 | 14 | 13 | 29 |
| KWA | Everest | 42 | - | - | - |
| OGI | Gallagher | - | 13 | 11 | 24 |
| OGI | Iba | - | 15 | 12 | 34 |
| KWA | Joe | 45 | 18 | 17 | 46 |
| PlainsGold | Langin | - | 11 | 14 | - |
| KWA | Larry | - | 21 | 16 | - |
| LCS | LCS Avenger | - | 17 | - | - |
| LCS | LCS Chrome | 37 | 20 | 16 | 38 |
| LCS | LCS Mint | 36 | 8 | 15 | 34 |
| LCS | LCS Pistol | 28 | 14 | 14 | 30 |
| OGI | Lonerider | 33 | 13 | 11 | 25 |
| Dyna-Gro | Long Branch | - | 20 | 14 | 35 |
| OGI | NF 101 | - | 9 | - | 25 |
| KWA | Oakley CL | - | 20 | 16 | - |
| OGI | Ruby Lee | 51 | 17 | 14 | 32 |
| OGI | Smith's Gold | 47 | 19 | 16 | 29 |
| OGI | Spirit Rider | - | 10 | - | - |
| OGI | Stardust | 50 | 11 | - | - |
| AgriPro | SY Achieve CL2 | - | 10 | 9 | - |
| AgriPro | SY Benefit | 40 | 7 | - | 30 |
| AgriPro | SY Flint | 33 | 13 | - | 35 |
| AgriPro | SY Grit | - | 15 | 11 | 27 |
| AgriPro | SY Monument | 45 | - | 16 | - |
| AgriPro | SY Rugged | - | 14 | 16 | 30 |
| LCS | T158 | 44 | 12 | 13 | 30 |
| Watley | TAM 112 | - | 20 | 15 | - |
| AGESCO | TAM 114 | - | 4 | 11 | - |
| Watley | TAM 204 | 38 | 10 | 11 | 20 |
| WestBred | WB4269 | 57 | 27 | - | - |
| WestBred | WB4303 | 19 | 22 | 11 | 31 |
| WestBred | WB4458 | 34 | 12 | 12 | 27 |
| WestBred | WB4515 | 35 | 24 | - | - |
| WestBred | WB4721 | - | 19 | 18 | 39 |
| WestBred | WB-Grainfield | 37 | 11 | 13 | 42 |
| WestBred | Winterhawk | - | 17 | 14 | - |
| KWA | Zenda | 42 | 9 | - | - |
| OSU Experimentals | |||||
| OCW03S580S-8F | 36 | - | - | - | |
| OCW04S717T-6W | 49 | 7 | - | 29 | |
| OCW05S616T-2 | 43 | - | 6 | 28 | |
| OK12206-2 | 41 | 5 | 6 | 20 | |
| OK12716 | - | 20 | 9 | 35 | |
| OK12D22004-016 | 51 | - | - | - | |
| OK13209 | - | 23 | - | 33 | |
| OK13621 | - | 11 | 8 | - | |
| OK14319 | 45 | - | - | - | |
| OK14438 | 57 | - | - | - | |
| OK14P212 | - | 22 | 11 | - | |
| OK168513 | - | - | 11 | - | |
| Mean | 41 | 15 | 13 | 31 | |
| LSD (0.05) | 7 | 7 | 6 | 6 |
| Source | Variety | Apache Fungicide | Balko | Buffalo | Cherokee |
|---|---|---|---|---|---|
| AGESCO | AG Gallant | - | - | - | - |
| AGESCO | AG Icon | 35 | - | - | - |
| AgriMAXX | AM Eastwood | - | - | - | - |
| OGI | Bentley | 35 | 40 | 37 | 32 |
| AgriPro | Bob Dole | - | - | - | 30 |
| CROPLAN | CP78-26 | - | - | - | - |
| OGI | Doublestop CL Plus | 36 | 33 | 28 | 34 |
| OGI | Duster | 29 | 33 | - | 25 |
| KWA | Everest | - | - | - | - |
| OGI | Gallagher | 25 | 37 | 27 | 22 |
| OGI | Iba | 33 | 24 | 31 | 31 |
| KWA | Joe | 45 | 44 | 36 | 34 |
| PlainsGold | Langin | - | 24 | 34 | 36 |
| KWA | Larry | - | 46 | 29 | 37 |
| LCS | LCS Avenger | - | - | - | - |
| LCS | LCS Chrome | 36 | 30 | 35 | 32 |
| LCS | LCS Mint | 36 | 40 | - | 30 |
| LCS | LCS Pistol | 32 | 43 | - | 30 |
| OGI | Lonerider | 25 | 35 | 27 | 22 |
| Dyna-Gro | Long Branch | 37 | 45 | - | 25 |
| OGI | NF 101 | 24 | - | - | - |
| KWA | Oakley CL | - | 51 | 32 | 29 |
| OGI | Ruby Lee | 29 | 30 | - | 25 |
| OGI | Smith's Gold | 33 | 32 | 27 | 23 |
| OGI | Spirit Rider | - | - | - | |
| OGI | Stardust | - | - | - | |
| AgriPro | SY Achieve CL2 | - | - | 20 | |
| AgriPro | SY Benefit | 32 | - | - | |
| AgriPro | SY Flint | 36 | - | - | |
| AgriPro | SY Grit | 30 | 24 | 26 | 31 |
| AgriPro | SY Monument | - | 33 | - | 37 |
| AgriPro | SY Rugged | 31 | 37 | 32 | 24 |
| LCS | T158 | 31 | 35 | - | 21 |
| Watley | TAM 112 | - | 25 | - | 37 |
| AGESCO | TAM 114 | - | 34 | 26 | 34 |
| Watley | TAM 204 | 22 | 31 | - | 27 |
| WestBred | WB4269 | - | - | - | - |
| WestBred | WB4303 | 28 | 34 | - | 24 |
| WestBred | WB4458 | 28 | 37 | - | 33 |
| WestBred | WB4515 | - | - | - | - |
| WestBred | WB4721 | 35 | 30 | 34 | 33 |
| WestBred | WB-Grainfield | 41 | 36 | - | 32 |
| WestBred | Winterhawk | - | 38 | 34 | 27 |
| KWA | Zenda | - | - | - | - |
| OSU Experimentals | |||||
| OCW03S580S-8F | - | - | - | - | |
| OCW04S717T-6W | 27 | - | - | 24 | |
| OCW05S616T-2 | 27 | 37 | 28 | 27 | |
| OK12206-2 | 19 | 37 | 28 | 17 | |
| OK12716 | 34 | 46 | 33 | 31 | |
| OK12D22004-016 | - | 41 | - | - | |
| OK13209 | 32 | - | - | - | |
| OK13621 | - | 35 | 24 | 28 | |
| OK14319 | - | - | - | - | |
| OK14438 | - | - | - | - | |
| OK14P212 | - | 32 | 39 | 34 | |
| OK168513 | - | 38 | 31 | - | |
| Mean | 31 | 36 | 31 | 29 | |
| 1 | LSD (0.05) | 5 | 6 | 7 | 11 |
| Source | Variety | Chickasha | Chickasha IWM | Goodwell Irrigated | Homestead |
|---|---|---|---|---|---|
| AGESCO | AG Gallant | 51 | 77 | 72 | - |
| AGESCO | AG Icon | 52 | 68 | 87 | - |
| AgriMAXX | AM Eastwood | 50 | 74 | 74 | - |
| OGI | Bentley | 48 | 80 | 75 | 14 |
| AgriPro | Bob Dole | 57 | 75 | 78 | 13 |
| CROPLAN | CP78-26 | 62 | 67 | 82 | - |
| OGI | Doublestop CL Plus | 55 | 71 | 75 | 13 |
| OGI | Duster | 49 | 66 | 91 | 14 |
| KWA | Everest | - | - | - | - |
| OGI | Gallagher | 64 | 78 | 88 | 17 |
| OGI | Iba | 65 | 74 | 81 | 22 |
| KWA | Joe | 58 | 77 | 87 | 13 |
| PlainsGold | Langin | 59 | 71 | 81 | - |
| KWA | Larry | 63 | 76 | 85 | 12 |
| LCS | LCS Avenger | 48 | 59 | 84 | - |
| LCS | LCS Chrome | 50 | 58 | 80 | 10 |
| LCS | LCS Mint | 53 | 74 | 83 | 16 |
| LCS | LCS Pistol | 47 | 61 | 87 | 15 |
| OGI | Lonerider | 63 | 78 | 90 | 15 |
| Dyna-Gro | Long Branch | 35 | 52 | 96 | - |
| OGI | NF 101 | 56 | 70 | 53 | - |
| KWA | Oakley CL | 55 | 64 | 82 | - |
| OGI | Ruby Lee | 60 | 73 | 78 | 13 |
| OGI | Smith's Gold | 63 | 81 | 82 | 15 |
| OGI | Spirit Rider | 57 | 77 | 80 | 15 |
| OGI | Stardust | 57 | 79 | 73 | 17 |
| AgriPro | SY Achieve CL2 | 56 | 69 | 70 | 15 |
| AgriPro | SY Benefit | 52 | 75 | 80 | 20 |
| AgriPro | SY Flint | 55 | 74 | 83 | 17 |
| AgriPro | SY Grit | 58 | 80 | 84 | 15 |
| AgriPro | SY Monument | - | - | 90 | 16 |
| AgriPro | SY Rugged | 57 | 67 | 78 | - |
| LCS | T158 | 61 | 75 | 70 | 11 |
| Watley | TAM 112 | 52 | 67 | 79 | - |
| AGESCO | TAM 114 | 67 | 81 | 92 | - |
| Watley | TAM 204 | 40 | 62 | 80 | 13 |
| WestBred | WB4269 | 63 | 78 | 80 | - |
| WestBred | WB4303 | 46 | 72 | 85 | 16 |
| WestBred | WB4458 | 59 | 77 | 83 | 13 |
| WestBred | WB4515 | 60 | 74 | 67 | 17 |
| WestBred | WB4721 | 53 | 71 | 83 | 14 |
| WestBred | WB-Grainfield | 56 | 79 | 87 | 10 |
| WestBred | Winterhawk | 60 | 78 | 85 | - |
| KWA | Zenda | 59 | 71 | 77 | 10 |
| OSU Experimentals | |||||
| OCW03S580S-8F | - | - | - | - | |
| OCW04S717T-6W | 73 | 75 | - | 8 | |
| OCW05S616T-2 | 64 | 73 | 75 | 21 | |
| OK12206-2 | 62 | 74 | 73 | 10 | |
| OK12716 | 51 | 69 | 78 | 13 | |
| OK12D22004-016 | 71 | 77 | 81 | - | |
| OK13209 | 55 | 70 | - | 10 | |
| OK13621 | 66 | 73 | 77 | - | |
| OK14319 | - | - | - | - | |
| OK14438 | - | - | - | - | |
| OK14P212 | - | - | 80 | 15 | |
| OK168513 | - | - | 74 | - | |
| Mean | 57 | 72 | 80 | 14 | |
| LSD (0.05) | 10 | 9 | 12 | 7 |
Summary 2: 2017-2018 Oklahoma Wheat Variety Performance Tests Summary
grain yield (bu/ac)+
| Source | Variety | Hooker | Keyes | Kildare | Kingfisher |
|---|---|---|---|---|---|
| AGESCOO | AGG Gallant | - | - | - | - |
| AGESCOO | AGG Icon | - | - | - | - |
| AgriMAXXX | AM Eastwood | - | - | - | - |
| OGII | Bentley | 38 | 35 | 42 | 20 |
| AgriProo | Bob Dole | - | - | 36 | - |
| CROPLANN | CP78-26 | - | - | - | - |
| OGII | Doublestop CL Plus | 35 | 31 | 43 | 8 |
| OGII | Duster | 37 | 30 | 31 | 13 |
| KWAA | Everest | - | - | - | - |
| OGII | Gallagher | 32 | 26 | 26 | 14 |
| OGII | Iba | 33 | 29 | 36 | 13 |
| KWAA | Joe | 38 | 36 | 43 | 9 |
| PlainsGoldd | Langinn | 45 | 32 | - | - |
| KWAA | Larry | 35 | 29 | - | 11 |
| LCSS | LCSS Avenger | - | - | - | - |
| LCSS | LCSS Chrome | 33 | 23 | 42 | 13 |
| LCSS | LCSS Mint | 41 | 34 | 39 | 13 |
| LCSS | LCSS Pistol | 36 | 26 | - | 14 |
| OGII | Loneriderr | 34 | 28 | - | 8 |
| Dyna-Gro | Long Branch | 42 | 35 | - | - |
| OGII | NF 101 | - | - | - | - |
| KWAA | Oakley CL | 36 | 35 | - | - |
| OGII | Ruby Lee | 37 | 28 | 37 | 18 |
| OGII | Smith's Gold | 34 | 27 | 31 | 10 |
| OGII | Spirit Rider | - | - | 34 | - |
| OGII | Stardust | - | - | 35 | - |
| AgriProo | SYY Achieve CL2 | - | - | 34 | 10 |
| AgriProo | SYY Benefit | - | - | 32 | 9 |
| AgriProo | SYY Flint | - | - | - | 8 |
| AgriProo | SYY Grit | 31 | 26 | - | 6 |
| AgriProo | SYY Monument | 40 | 34 | 38 | 11 |
| AgriProo | SYY Rugged | 30 | 31 | - | 10 |
| LCSS | T158 | 32 | 27 | - | 12 |
| Watleyy | TAM 112 | 36 | 34 | - | - |
| AGESCOO | TAM 114 | 33 | 29 | - | - |
| Watleyy | TAM 204 | 27 | 21 | 24 | 12 |
| WestBredd | WB4269 | - | - | 42 | - |
| WestBredd | WB4303 | 30 | 26 | 32 | 8 |
| WestBredd | WB4458 | 38 | 25 | 35 | 7 |
| WestBredd | WB4515 | - | - | - | 9 |
| WestBredd | WB4721 | 31 | 27 | - | - |
| WestBredd | WBB-Grainfield | 40 | 33 | 38 | 10 |
| WestBredd | Winterhawkk | 36 | 30 | - | - |
| KWA | Zenda | - | - | 28 | - |
| OSUExperimentalss | |||||
| OCW03S580S-8F | - | - | - | - | |
| OCW04S717T-6W | - | - | - | 5 | |
| OCW05S616T-2 | 31 | 34 | 31 | 7 | |
| OK12206-2 | 32 | - | - | 7 | |
| OK12716 | 36 | 30 | 38 | 10 | |
| OK12D22004-016 | - | - | 32 | 11 | |
| OK13209 | - | - | - | 9 | |
| OK13621 | 36 | - | - | 8 | |
| OK14319 | - | - | - | 7 | |
| OK14438 | - | - | - | - | |
| OK14P212 | 37 | 34 | - | - | |
| OK168513 | 34 | 32 | - | - | |
| Mean | |||||
| LSD (0.05) |
| Source | Variety | Lahoma | Lahoma Fungicide | Lamont | Marshall Dual-Purpose |
|---|---|---|---|---|---|
| AGESCOO | AGG Gallant | 42 | 42 | - | - |
| AGESCOO | AGG Icon | 34 | 29 | - | 15 |
| AgriMAXXX | AM Eastwood | 26 | 26 | - | - |
| OGII | Bentley | 48 | 48 | 33 | 14 |
| AgriProo | Bob Dole | 20 | 19 | 33 | 13 |
| CROPLANN | CP78-26 | 41 | 39 | - | - |
| OGII | Doublestop CL Plus | 35 | 33 | 36 | 15 |
| OGII | Duster | 26 | 25 | 22 | 14 |
| KWAA | Everest | - | - | - | - |
| OGII | Gallagher | 35 | 35 | 19 | 8 |
| OGII | Iba | 30 | 27 | 30 | 15 |
| KWAA | Joe | 32 | 35 | 30 | 16 |
| PlainsGoldd | Langinn | 39 | 40 | - | - |
| KWAA | Larry | 30 | 27 | 35 | - |
| LCSS | LCSS Avenger | 29 | 34 | - | - |
| LCSS | LCSS Chrome | 35 | 30 | 31 | 16 |
| LCSS | LCSS Mint | 37 | 36 | 34 | - |
| LCSS | LCSS Pistol | 35 | 32 | 23 | - |
| OGII | Loneriderr | 30 | 27 | 21 | - |
| Dyna-Gro | Long Branch | 39 | 45 | - | 19 |
| OGII | NF 101 | 22 | 24 | - | - |
| KWAA | Oakley CL | 32 | 30 | - | - |
| OGII | Ruby Lee | 45 | 47 | 31 | 13 |
| OGII | Smith's Gold | 35 | 34 | 24 | 12 |
| OGII | Spirit Rider | 33 | 35 | 26 | - |
| OGII | Stardust | 37 | 28 | 28 | 10 |
| AgriProo | SYY Achieve CL2 | 27 | 24 | 28 | 5 |
| AgriProo | SYY Benefit | 33 | 32 | 24 | - |
| AgriProo | SYY Flint | 28 | 21 | 33 | - |
| AgriProo | SYY Grit | 22 | 19 | 28 | 18 |
| AgriProo | SYY Monument | 25 | 23 | - | - |
| AgriProo | SYY Rugged | 28 | 25 | - | 14 |
| LCSS | T158 | 38 | 39 | 26 | 17 |
| Watleyy | TAM 112 | 40 | 41 | - | - |
| AGESCOO | TAM 114 | 29 | 30 | - | - |
| Watleyy | TAM 204 | 33 | 27 | 16 | 14 |
| WestBredd | WB4269 | 49 | 53 | 35 | 15 |
| WestBredd | WB4303 | 28 | 27 | 31 | 9 |
| WestBredd | WB4458 | 31 | 29 | 26 | - |
| WestBredd | WB4515 | 35 | 33 | 34 | 18 |
| WestBredd | WB4721 | 39 | 40 | - | 15 |
| WestBredd | WBB-Grainfield | 28 | 27 | 26 | - |
| WestBredd | Winterhawkk | 27 | 28 | - | - |
| KWA | Zenda | 27 | 30 | 30 | - |
| OSUExperimentalss | |||||
| OCW03S580S-8F | - | - | - | - | |
| OCW04S717T-6W | 23 | 23 | 25 | 9 | |
| OCW05S616T-2 | 22 | 23 | 22 | 10 | |
| OK12206-2 | 20 | 20 | - | 11 | |
| OK12716 | 31 | 29 | 24 | 17 | |
| OK12D22004-016 | 28 | 25 | 26 | - | |
| OK13209 | 32 | 32 | 33 | - | |
| OK13621 | 31 | 31 | 25 | - | |
| OK14319 | 25 | 21 | - | 14 | |
| OK14438 | - | - | - | - | |
| OK14P212 | - | - | - | 17 | |
| OK168513 | - | - | - | - | |
| Mean | 32 | 31 | 28 | 14 | |
| LSD (0.05) | 8 | 8 | 6 | 6 |
| Source | Variety | Marshall Grain-Only | Thomas | Union City | Walters |
|---|---|---|---|---|---|
| AGESCOO | AGG Gallant | - | - | - | - |
| AGESCOO | AGG Icon | 38 | - | - | - |
| AgriMAXXX | AM Eastwood | - | - | - | - |
| OGII | Bentley | 37 | 23 | 27 | 63 |
| AgriProo | Bob Dole | 39 | - | - | - |
| CROPLANN | CP78-26 | - | - | - | - |
| OGII | Doublestop CL Plus | 29 | 22 | 26 | 67 |
| OGII | Duster | 37 | 13 | 13 | 63 |
| KWAA | Everest | - | - | - | - |
| OGII | Gallagher | 32 | 15 | 7 | 56 |
| OGII | Iba | 37 | 14 | 23 | 68 |
| KWAA | Joe | 38 | 24 | - | 72 |
| PlainsGoldd | Langinn | - | - | - | - |
| KWAA | Larry | - | 17 | - | - |
| LCSS | LCSS Avenger | - | - | - | - |
| LCSS | LCSS Chrome | 35 | 16 | 21 | 69 |
| LCSS | LCSS Mint | - | 23 | 12 | 52 |
| LCSS | LCSS Pistol | - | 15 | 15 | 57 |
| OGII | Loneriderr | - | 16 | 19 | 60 |
| Dyna-Gro | Long Branch | 30 | 21 | - | 47 |
| OGII | NF 101 | - | - | 8 | 43 |
| KWAA | Oakley CL | - | - | - | - |
| OGII | Ruby Lee | 37 | 17 | 15 | 51 |
| OGII | Smith's Gold | 36 | 18 | 11 | 62 |
| OGII | Spirit Rider | - | - | - | - |
| OGII | Stardust | 36 | - | - | - |
| AgriProo | SYY Achieve CL2 | 39 | 14 | - | - |
| AgriProo | SYY Benefit | - | 11 | 18 | 50 |
| AgriProo | SYY Flint | - | 17 | 21 | 59 |
| AgriProo | SYY Grit | 36 | 17 | 21 | 62 |
| AgriProo | SYY Monument | - | 18 | - | - |
| AgriProo | SYY Rugged | 33 | 18 | 12 | 65 |
| LCSS | T158 | 35 | 13 | 17 | 63 |
| Watleyy | TAM 112 | - | - | - | - |
| AGESCOO | TAM 114 | - | - | - | - |
| Watleyy | TAM 204 | 40 | 15 | 10 | - |
| WestBredd | WB4269 | 44 | - | - | - |
| WestBredd | WB4303 | 41 | 17 | 13 | 67 |
| WestBredd | WB4458 | - | 19 | 13 | 45 |
| WestBredd | WB4515 | 39 | 28 | 18 | - |
| WestBredd | WB4721 | 35 | 16 | 20 | 56 |
| WestBredd | WBB-Grainfield | - | 17 | 26 | 55 |
| WestBredd | Winterhawkk | - | - | - | - |
| KWA | Zenda | - | - | - | - |
| OSUExperimentalss | |||||
| OCW03S580S-8F | - | - | - | - | |
| OCW04S717T-6W | 34 | - | - | - | |
| OCW05S616T-2 | 39 | 11 | 9 | 63 | |
| OK12206-2 | 32 | 9 | 8 | - | |
| OK12716 | 32 | 17 | 18 | 68 | |
| OK12D22004-016 | - | 17 | - | - | |
| OK13209 | - | 17 | 17 | 59 | |
| OK13621 | - | - | 23 | - | |
| OK14319 | 39 | - | - | - | |
| OK14438 | - | - | 21 | - | |
| OK14P212 | 40 | 14 | - | - | |
| OK168513 | - | - | - | ||
| Mean | 36 | 17 | 16 | 59 | |
| LSD (0.05) | 5 | 6 | 6 | 10 |
Brett Carver
Plant and Soil Sciences

