Understanding Free Sulfur Dioxide (FSO₂) in Wine
Free SO₂ is the amount of available sulfur dioxide in wine to help protect against oxidation and microbial spoilage. Free SO₂ acts as a preservative to inhibit the growth of microorganisms and to protect against oxidation, as oxidation and microorganisms will spoil wine. Free SO₂ is often measured in parts per million (ppm) using either the aspiration-oxidation method or the ripper method. These methods require basic knowledge of chemistry that is essential for good winemaking.
Free SO₂ in wine will become Bound SO₂ in wine over time. This is because free sulfur dioxide is used up (binds) as it protects against oxidation and spoilage microbes. Bound SO₂ does not protect against oxidation or spoilage microbes but remains permanently present in the wine. Increasing Free SO₂ levels by sulfur additions, such as potassium metabisulfite (KMBS), is only a temporary fix as Bound SO₂ levels will only increase over time. Therefore, it is extremely important to have a sulfur dioxide management regime during winemaking and to continuously monitor sulfur dioxide levels until the wine is bottled.
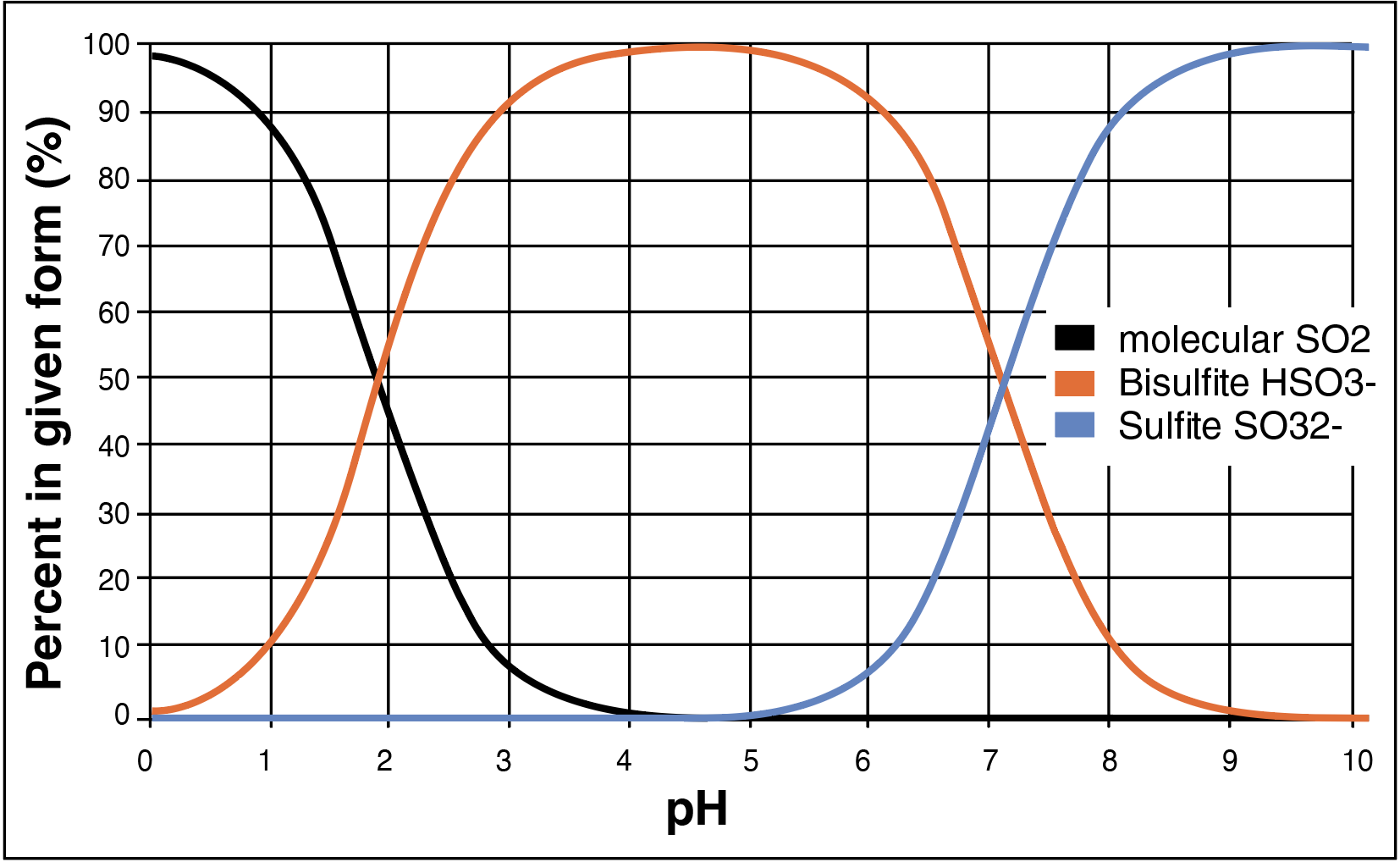
The amount of free molecular SO₂ available to protect against oxidation and spoilage microbes is dependent upon the pH (acidity) of the wine. This is because sulfur dioxide exists in three different forms: SO₂ (molecular form), HSO₃¹⁻ (bisulfite form), and SO₃²⁻ (sulfite form). The bisulfite form is more prevalent at wine pH, but as the pH is lowered, i.e., as the wine becomes more acidic, the molecular form becomes more prevalent. It is the molecular form that is best suited to offer protection in wine.
Chart 1 shows the percentage of sulfur dioxide in each form at different pH ranges. Wine mostly exists between a pH of 3.0 – 4.0 where less than 10% of free sulfur dioxide exists in the molecular form while the rest exists in the bisulfite form. The sulfite form (SO32-) is rarely, if ever, discussed for winemaking. Free SO₂ is almost completely in the bisulfite form at a wine pH above 4.0, and this form is far less effective in offering any type of protection. Thus, adjusting wine pH prior to an SO₂ addition is sometimes necessary.
Chart 1. The three different forms of free sulfur dioxide at varying pH.
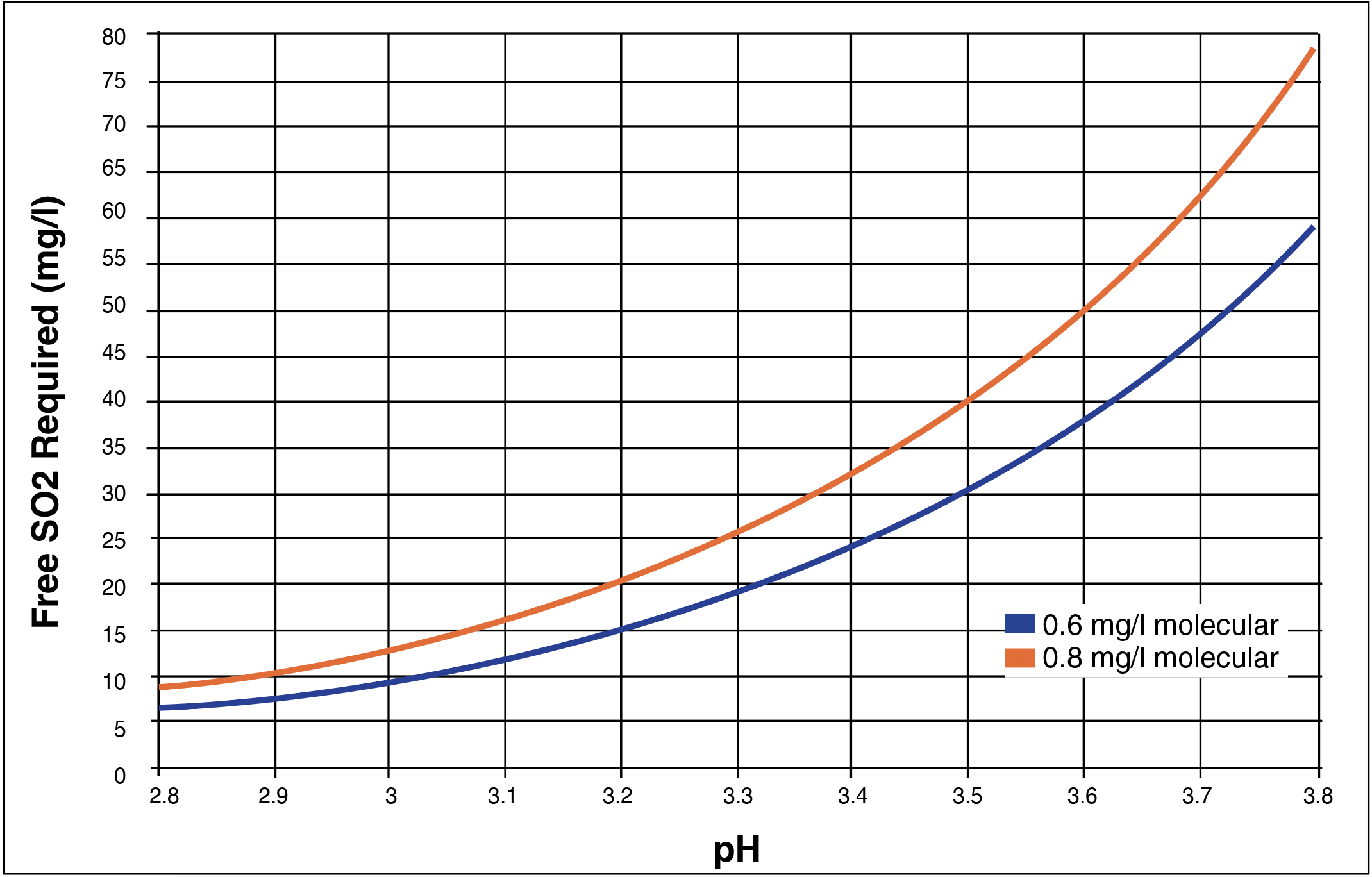
Since it is most desirable to have the molecular form of Free SO₂ present in wine, it is also important to understand the concentration of the molecular form of Free SO₂ obtained when making a KMBS addition to wine at a specific pH. For example, adding 50 ppm of KMBS to wine with a pH of 3.6 will only offer 0.8 ppm of molecular SO₂ for protection, while a wine with a pH of 3.2 will require the addition of only 20 ppm of KMBS to have that same 0.8 ppm of molecular SO₂ present for protection. Chart 2 and Chart 3 help to explain this phenomenon. This variable relationship among Free SO₂, molecular SO₂ and wine pH can quickly become confusing, especially if there is already Free SO₂ present in wine when making a KMBS addition. Please remember to always double check KMBS calculations before making an addition, record every addition, have a Free SO₂ management regime, and continuously monitor Free SO₂ levels in wine until bottling. When making an SO₂ addition, it is important to thoroughly stir and mix the wine to fully incorporate the sulfur dioxide.
Chart 2. The concentration of molecular SO₂ within the concentration of Free SO₂ at various wine pH points. Please note that milligrams per Liter (mg/L) is interchangeable with parts per million (ppm). (1mg/L = 1ppm).
| pH of Wine | % as Molecular SO₂ | Free SO₂ Concentration (ppm) for 0.8 ppm Molecular SO₂ |
|---|---|---|
| 3.00 | 6.06 | 14 |
| 3.10 | 4.88 | 18 |
| 3.20 | 3.91 | 22 |
| 3.30 | 3.13 | 28 |
| 3.40 | 2.51 | 35 |
| 3.50 | 2.00 | 44 |
| 3.60 | 1.60 | 55 |
| 3.70 | 1.27 | 69 |
| 3.80 | 1.01 | 87 |
| 3.90 | 0.81 | 109 |
| 4.0 | 0.64 | 125 |
Chart 3. The numerical value as a percentage of molecular SO₂ within the concentration of Free SO₂ at a specific wine pH.
Calculations for the addition of KMBS:
•(Desired ppm to add x Volume in Liters) / 0.57 = grams of KMBS to add
Example:
(50 ppm x 225 Liters) / 0.57 = 19.7g of KMBS to add (0.05 x 225) / 0.57 = 19.7g
Recommended FSO₂ Range in Wine:
15 ppm - 40 ppm