Soil pH and Buffer Index
- Jump To:
- High pH
- Low pH
- How to Use the Buffer Index
The purpose of this fact sheet is to explain the general concept of soil pH and Buffer Index and to show how these soil test values are used in making Aglime recommendations. Soil pH is a very important soil chemical property because it strongly influences availability of plant nutrients in the soil and can have a drastic effect on crop production.
Crops vary in their tolerance, or ability to grow, in soil at very high and very low pH (see Table 1). A soil pH of 7.0 is neutral and is used as a reference to categorize soils as acidic (pH less than 7.0) or basic (pH above 7.0). For the production of most crops a slightly acid soil, about pH 6.5, is most desirable.
Table 1. pH Preference of Common Field Crops.
| pH Range | ||
|---|---|---|
| Legumes | ||
| Cowpeas, Crimson Clover, Soybeans, and Vetch | 5.5-7.0 | |
| Alsike, Red and White, (Ladino) Clovers, and Arrowleaf Clover | 6.0-7.0 | |
| Alfalfa and Sweet Clover | 6.3-7.5 | |
| Non-Legumes | ||
| Fescue and Weeping Lovegrass | 4.5-7.0 | |
| Buckwheat | 5.0-6.5 | |
| Sorghum, Sudan and Wheat | 5.5-7.0 | |
| Bermuda | 5.7-7.0 | |
| Barley | 6.3-7.0 |
High pH
The soil pH is seldom too high (basic or alkaline) to interfere with crop production. However, when crop production declines due to high soil pH, it is usually because the pH is 8.5 or higher and water movement into the soil is drastically reduced. This problem can be corrected if the soil has good internal drainage, or it can be provided for, and the alkali salts such as sodium can be leached out. The leaching will only be possible after the required amount of gypsum has been applied. For more information on alkali soils see Fact Sheet PSS-2226, “Reclaiming Slick-Spots and Salty Soils.”
Low pH
Well-drained, productive soils under good management will slowly become acidic because acidity is a natural result of high crop production. In addition, the amount of rainfall, soil texture and reserve of basic minerals in the soil will influence the time required for neutral and basic soils to become acidic.. It will take some of our productive Oklahoma panhandle soils more than 100 years to become acidic; while some soils in northcentral Oklahoma have become acidic in just the last 20 to 30 years. Irrigated soils adjust with time to the pH of the irrigation water. Extremely acid soils may not be productive because of the presence of increased amounts of toxic elements such as aluminum and manganese.
The Soil Reservoir
The soil is often regarded as a reservoir of nutrients and water for plant growth. In addition, the soil solids are a reservoir of basic or acidic material, which regulates soil pH. It is this reservoir feature of soils that makes them so resistant to rapid change in spite of large annual additions of fertilizer materials and mineral removals by crop production. The buffer capacity of soils is the capacity of soils to resist change. In relation to soil pH, the soil Buffer Index is a measure of the soil reservoir of basic material, which will serve to resist change in soil pH. By comparison, soil pH measures the current acid or basic condition of the soil, as the plant experiences it, and provides no information about the soil acid or basic reservoir. The relationship between these two soil properties, soil texture and liming, is illustrated in Figures 1 and 2.
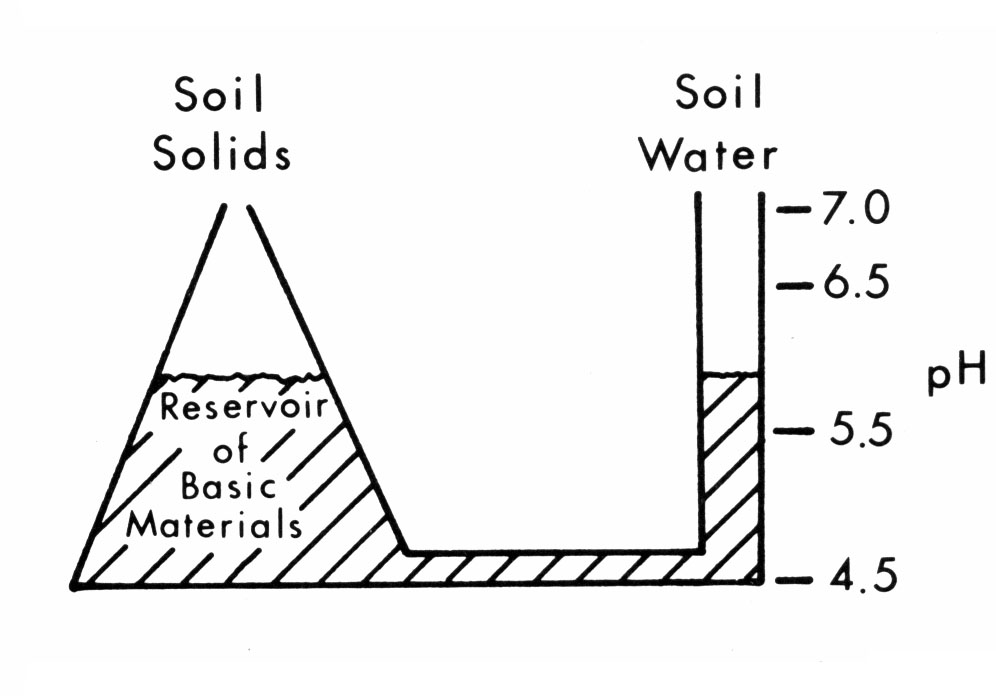
Figure 1. The relationship of basic materials in soil solids to pH of the soil.
Figure 2. Reservoirs of soil solids in clayey vs. sandy soils.
Buffering
Figure 1 shows that soil pH is related to the level of bases (calcium, magnesium, potassium, etc.) or basic materials in the soil water. As crops remove bases from soil water in the reservoir on the right, bases from the large reservoir of soil solids on the left move to the soil solution and replenish the supply. Because of this relationship and the large reserve of bases from soil solids, the pH does not change much from month to month or even year to year. Also, since the large reservoir on the left is shaped like a pyramid, pH can be changed more easily by liming at pH near 6 than in the very acid pH 4.5 to 5.5 range.
Figure 2 shows the influence of soil organic matter and texture. Both soils have a pH of 4.3 and are too acidic for efficient crop production. In order to provide a more favorable pH, the soils must each be limed. The amount of lime required will depend on the size of the large reservoirs and how empty they are of bases.
From these diagrams it is easy to understand why it takes much more lime to raise the pH of a clayey soil with its large reservoir than it does for a sandy soil and its small reservoir. Also, because the reservoir of sandy soil is small, if acidifying conditions are equal sandy soil will tend to become acid more rapidly and need to be limed more frequently than clayey soil.
The Soil Test
Buffer Index (BI) measured in the laboratory, as a part of the Oklahoma State University soil test, is an indirect estimate of the soil reservoir size for storing basic material. Because the test involves adding basic (lime-like) material to soils of pH less than 6.5 and then measuring pH again, the BI pH is larger when the reservoir is small. The two soils illustrated in Figure 2 need to be limed. The Pond Creek Silt Loam soil would have a BI value of about 6.2. About 4.2 tons of ECCE lime would be required to raise the soil pH to 6.8. The sandy soil, having the same soil pH, would have a BI value of about 6.5 and require only 2.4 tons of ECCE lime. The field calibration for BI and lime requirement is provided in Table 2.
Table 2. Tons of ECCE* Lime Required to Raise Soil pH of a 6-7 Inch Furrow Slice to pH 6.8 or 6.4.
| Buffer Index | Lime Required (pH 6.8) | Lime Required (pH 6.4) | |
|---|---|---|---|
| over 7.1 | none | none | |
| 7.1 | 0.5 | none | |
| 7.0 | 0.7 | none | |
| 6.9 | 1.0 | none | |
| 6.8 | 1.2 | 0.7 | |
| 6.7 | 1.4 | 1.2 | |
| 6.6 | 1.9 | 1.7 | |
| 6.5 | 2.5 | 2.2 | |
| 6.4 | 3.1 | 2.7 | |
| 6.3 | 3.7 | 3.2 | |
| 6.2 | 4.2 | 3.7 |
* Effective calcium carbonate equivalent guaranteed by lime vendor.
Tons material required = Tons ECCE required ÷% ECCE of the lime x 100
How to Use the Buffer Index
Considering a soil test result of pH 5.8 and Buffer Index 6.8, where establishment of alfalfa is intended the following steps are taken to determine lime requirement.
First, the soil test pH of 5.8 is compared to the preferred pH for alfalfa in Table 1. Since the soil pH 5.8 is below the lowest pH in the preferred range, lime must be added to raise the pH to the desired level.
The amount of lime required is determined from Table 2 by locating the Buffer Index value of 6.8 in the left hand column and matching it to the number directly across from it (dashed line) under the middle column of numbers. In this case, 1.2 tons of ECCE lime would be required.
If the intended crop were wheat instead of alfalfa, no lime would be required this year because Table 1 shows that pH 5.8 is satisfactory for wheat production. Since the pH is satisfactory for wheat, the lime requirement would not be reported, even though the Buffer Index was measured. It would be important to regularly test this soil, especially if it were sandy, so lime could be applied before the soil became seriously acid (below pH 5.0) for wheat production, especially grazing is considered.
For situations where the soil has become extremely acid, as illustrated in Figure 2, for wheat production it is possible to apply less than the total lime required to raise the soil pH to 6.8. For example, of the 4.2 tons total required to raise the pH of the Pond Creek soil to 6.8, about 1.2 tons would bring the pH out of the danger zone and allow near normal production for three to five years (see OSU Fact Sheet PSS-2240, “Managing Acid Soils for Wheat Production”). With this approach soil pH should be closely monitored and more lime should be planned in the next year or two.
Remember, Buffer Index is only used as a guide for how much lime should be added to an acid soil when it is necessary to raise soil pH.
Hailin Zhang
Director, Soil, Water and Forage Analytical Laboratory

