Per - and Polyfluoroalkyl Substance (PFAS) - Potential Risk to Rural Groundwater Systems
- Jump To:
- Introduction
- What are PFAS?
- Public Health Risk
- Where are they Found?
- Why are they Used?
- Environmental Concern
- Rural Drinking Water Concern
- PFAS Testing Results for Oklahoma Public Water Systems
- Impacts of the EPA PFAS Rule
- In-Home Treatment Option for PFAS
- Rule for Privately Owned Drinking Water Systems
- Path to Exposure
- How to Protect Yourself
- PFAS Funding from the EPA
- Additional PFAS Resources
- References and Additional Information
Introduction
Hundreds of everyday consumer products are manufactured with highly toxic fluorinated chemicals called Per – and Polyfluoroalkyl Substances (PFAS). Classified as contaminants of emerging concern by the U.S. Environmental Protection Agency (EPA), PFAS have been in use since the 1940s but are gaining more attention recently as a result of their known risk to human health and the environment. These chemicals build up in our bodies and break down extremely slowly in the environment. Despite their associated risks, PFAS continues to be used in many common daily consumer items including non-stick cookware, food packaging, cosmetics, and stain resistant and water repellant products. PFAS have been detected in surface water and groundwater in many areas across the United States, posing concern to rural and urban communities alike. Therefore, providing educational resources, particularly to rural and underserved communities, can play a significant role in helping to reduce the health risk associated with PFAS exposure, and to better safeguard individuals and families. Regardless of whether you live in a rural or urban community, this factsheet seeks to help you understand the basics of PFAS, including the most common ways people can be exposed to them, the associated health risks, and how to reduce or prevent exposure. This factsheet will also provide information on the recent (2024) EPA PFAS rule, point out public water systems across Oklahoma that have known PFAS contamination, and provide additional PFAS resources at the state and federal level.
What are PFAS?
Previously referred to as Perfluorochemicals (PFCs), PFAS are a large, complex and ever-expanding group of man-made fluorinated chemicals that are resistant to water, heat, and oil. These chemicals do not break down over time and are commonly referred to as “forever chemicals”. PFAS accumulate in the environment over time and can pose a significant health risk with repeated exposure. Below are common examples of PFAS:
- Perfluoro octane sulfonic acid (PFOS)
- Perfluorooctanoic acid (PFOA or C8)
- Perfluorononanoic acid (PFNA)
- Perfluorodecanoic acid (PFDeA or PDFA)
- Perfluorooctane sulfonamide (PFOSA or FOSA)
- Perfluoroheptanoic acid (PFHpA)
- Perfluorobutanesulfonic acid (PFBS)
- Perfluorobutyrate (PFBA)
- 2- (N-Methyl-perfluorooctane sulfonamido) acetic acid (MeFOSAA or Me-PFOSA-AcOH)
- 2- (N-Ethyl-perfluorooctane sulfonamido) acetic acid (Et-FOSAA or Et-PFOSA-AcOH)
- Perfluorohexane sulfonic acid (PFHxS)
- Hexafluoropropylene Oxide (HFPO Dimer Acid HFPO-DA, also known by the trade name GenX chemicals)
PFOA and PFOS are two of the most common PFAS chemicals used in industries across the world, including the US. These industries include the aerospace, automotive, construction, electronic, and military sectors. In 2002, U.S. manufacturers voluntarily ceased the production of PFOS, followed by the phase-out of PFOA in 2006. GenX and Perfluorobutane Sulfonic acid (PFBS), both members of a larger PFAS group, were used as replacements for PFOA and PFOS, respectively. However, emerging evidence has shown the persistence of GenX chemicals in the environment and its bioaccumulation in the human body with health effects (Xu et al., 2021).
Public Health Risk
Products containing PFAS are used in every home. According to past research by the Centers for Disease Control (CDC), approximately 98% of U.S. population has some amount of PFOS present in their blood serum (Brennan et al. 2021). PFAS enters the human body through the consumption of contaminated foods, migration of chemicals from food packaging or cookware, or inhaling PFAS contaminated air. Research has proven that dietary intake is the major exposure pathways to PFOS and PFOA, followed by drinking water consumption in humans (Domingo & Nadal, 2019). Even more concerning, Zheng et al. (2021) detected PFAS in breastmilk of nursing mothers. Constant exposure to PFAS chemicals may lead to significant health issues. Research has documented adverse health effects associated with exposure to PFAS. For example, if humans eat or drink food or water contaminated by PFAS, the chemicals are absorbed and have the tendency to accumulate in the human body leading to health problems. Several studies have linked PFAS chemicals to the health situations noted below (Macheka et al. 2022; Anderko & Pennea, 2020; Fletcher et al 2020):
- Low birth weight
- Reproductive Problems
- Preeclampsia
- Pregnancy induced hypertension
- Ulcerative colitis
- Thyroid disease
- Weakened childhood immunity
- Weight gain in children and dieting adults
- Increased cholesterol
- Endocrine disruption
- Cancer includes testicular, kidney, liver, and pancreatic cancer
- Reduces vaccine-induced immune protection in children. Some research studies suggest that immunotoxicity and developmental toxicity are some of the most sensitive endpoints for PFAS in children (National Toxicology Program)
Where are they Found?
PFAS can be found in water, air, soil, and food, as well as in everyday items in our homes and workplaces. Below are specific examples of products and sources where PFAS are commonly found.
Manufacturers and Industry Users
PFAS are widely used by both primary (who produce raw materials) and secondary (who convert these materials into final goods) manufacturers. Both in the U.S. and abroad, some primary manufacturing facilities produce PFAS while secondary manufacturing facilities use them to produce industrial and consumer products such as those described below.
- Commercial household products: PFAS have a unique quality to repel water and oil. Because of this, they are used in everyday consumer products including stain and water repellant fabrics, nonstick cookware, and products such as lubricants, paints, pizza boxes, popcorn bags, polishes, carpets, and cleaning products. These products make our everyday lives much easier; however, the convenience may come with significant health risks.
- Personal care products: Studies by the Danish Environmental Protection Agency in 2018 confirmed the presence of certain PFAS in cosmetics including some commonly used lotions, shaving cream, foundation, mascara, nail polish, eye liner and some personal care products like dental floss.
Food
According to the U.S. Food and Drug Administration (FDA), PFAS reaches the food chain through (1) migration from food packaging and (2) environmental contamination. Due to their grease and moisture-repellant properties, PFAS are commonly used in food packaging including fast food containers.
Living Organisms
PFAS are found in living organisms raised in an environment contaminated by PFAS including soil, water, or bio-solids (nutrient rich by-products of wastewater treatment).
Fluorinated Firefighting Foams
A significant source of PFAS (especially in military and aviation settings) includes aqueous film forming foam (AFFF) and other fluorinated Class B firefighting foams. AFFF is a widely documented source of PFAS in the environment and are released in large quantities at firefighting training areas as part of routine handling, fire suppression trainings and equipment testing, and during emergency response (Anderson et al., 2016).
Essential Uses
PFAS are widely used in essential applications including medicine, defense, and aerospace.
- Medical uses: Long-lasting materials are of great importance for the health and well-being of patients, thus making flu-oropolymers (a type of PFAS) valuable in the healthcare field. PFAS are extremely long-lasting and are chemically inert. They are used in surgical gowns and drapes to resist contamination, and in hospital floor coverings to allow aggressive cleaning to help reduce potential sources of infection. PFAS also have other uses in medical applications that are not as easily seen, including implantable medical device, stent grafts to repair cardiac issues, surgical meshes used in repairing hernias, heart patches used for reconstruction in critical situations associated with tissue attachment, catheter tubes and guide wires used in a variety of procedures, sterile container filters, needle retrieval systems, tracheostomies, and inhaler canister coatings.
- Defense uses: PFAS are widely used by the Department of Defense (DOD). The DOD started using PFAS in the 1970s with the use of AFFF for the purpose of fuel firefighting. AFFF has PFAS and may have PFOS and, in some formulations, PFOA, two chemicals of the larger class of PFAS.
- Aerospace uses: Fluoropolymers play a very important role in the aerospace industry. They are generally used to increase durability, provide heat and fire resistance, lower dielectric constants, and increase the reliability of wires, optical, and data transmission cables. This includes cable and wire insulation to improve signal quality for critical data transmission and increased durability. They are also used in feeder antennas to improve in-flight connectivity on wire networks, and in aircraft interior coating to reduce flame, fouling and ease aircraft interior cleaning.
Landfills
Landfills are commonly used for the disposal of solid waste from various sources including residential, commercial, and industrial sites. Generally, landfill workers do not use PFAS during operations; however, they often receive them in the form of daily discarded products such as cosmetics, food packaging, carpeting, outdoor clothing, paper products etc. When consumer products containing PFAS are thrown away, the chemicals themselves also end up in the waste site. As the materials break down, PFAS are released from waste either to landfill leachate (water soluble PFAS) or to landfill gas (neutral PFAS with low water solubility and relatively high vapor pressures). PFAS in landfill leachate has the tendency of affecting adjacent soil, groundwater, and surface water based on the structural integrity of the landfill. Some landfills divert leachate into wastewater treatment plants for treatment.
Sewers
Nearly every person has some amount of PFAS in their bodies due to environmental exposure. As a result, these chemicals are introduced into our sewer systems through urine and feces. Flushed industrial waste is another source of PFAS in the sewage system. PFAS can also reach the sewer systems when clothes containing the chemical are washed.
Water
PFAS may be found in water supplies including private drinking water wells, public water systems, lakes and ponds that are near places that make, dispose of, or use PFAS. In some communities, PFAS may find its way into the water supply either by seeping through the soil into groundwater or by runoff. You can learn more about your local water supply by requesting your city’s water quality report from the city or local government; however, these water quality reports do not currently include information on PFAS. This may change in the future. Also, watch out for drinking water health advisories from the Oklahoma Department of Environmental Quality (DEQ).
Why are they Used?
One might be wondering why PFAS are not completely banned or why we are still using them. PFAS has a unique quality of being chemically and thermally stable thus allowing products to be nonstick, water-repellant and stain resistant. The fact that PFAS are long-lasting and biologically and chemically inert makes them crucial components to many medical devices and applications On the other hand, PFAS do not break down easily, allowing them to build up in the human body over time with the possibility of interfering with bodily functions like hormone regulation and immune system response. Therefore, the exact properties that make PFAS harmful to humans and the environment are the same properties that make them useful in saving lives and making life easier.
Environmental Concern
PFAS enters and builds up in the environment through various pathways associated with their production, use, and disposal. Their stable chemical structure makes them valuable in consumer products, but also contributes to their persistence in the environment. Environmental concern for PFAS arises from their widespread detection, high degree of environmental stability and mobility, and suspected toxicological effects on humans and the environment. After decades of use, PFAS chemicals have contaminated our air, water, soil, and food. As a result of this, every Americans has at least some level of PFAS in their blood stream.
Rural Drinking Water Concern
Groundwater is a very useful resource across the U.S. and the world at large. It is one of Oklahoma’s most precious natural resources and oftentimes the sole source of drinking water for many communities, especially those in rural and remote areas. Oklahoma has twenty-two major groundwater basins with roughly 390 million acre-feet of water in storage of which only one-half of that amount recoverable (Boyer et al., 2017). The most common water source in the western half of the state is groundwater (OWRB, 2024). Many rural communities in Oklahoma rely on groundwater for agriculture and other daily use including drinking, cooking, and bathing purposes. However, groundwater is vulnerable to contamination and can be easily threatened by chemicals like PFAS and pollution. In the U.S., nearly all states have detected an elevated level of PFAS compounds in their drinking water (Evans et al., 2020). PFAS can make its way into drinking water when products containing them are used or spilled onto the ground or into waterways such as rivers or lakes. Once this chemical finds its way into the groundwater system, it can contaminate nearby private wells or drinking water. The presence of PFAS in drinking water is a significant issue of concern nationwide considering the health implications listed above. PFAS may be released into the atmosphere during the manufacturing process and then redeposited on land where they can affect surface water and groundwater. PFAS may also be discharged without treatment to wastewater treatment plants or landfills, and eventually be released into the environment by treatment systems that are not designed to treat PFAS.
EPA’s Plan to Safeguard Drinking Water Systems
On April 10, 2024, EPA, the lead Federal Agency responsible for protecting the U.S. environment and natural resources including its drinking water, disclosed their rule and national standards for PFAS. The goal for this rule making is ensure significant reduction of the level of PFAS in drinking water across the U.S.
The Rule
- The rule includes setting limits for five individual PFAS: PFOA, PFOS, PFNA, PFHxS, and HFPO-DA (also known as GenX Chemicals). Additionally, the rule sets a hazard index level for mixtures of two more of the following four PFAS: PFNA, PFHxS, HFPO-DA, and PFBS.
- The rule requires public water systems to monitor these PFAS, providing up to three years (by 2027) to complete initial monitoring, followed by ongoing compliance monitoring.
- Public water systems are to provide residents with information on the levels of these PFAS in their drinking water starting in 2027.
- The rule gave public water systems five years (by 2029) to implement solutions that reduce these PFAS if monitoring indicates that the drinking water exceeds the above MCLs.
- Beginning in 2029, public water systems that have PFAS in their drinking water, which violates one or more of these MCLs, must put in place control measures to reduce PFAS levels in their drinking water and must provide notification to the public of the violation.
| Chemical | Maximum Contaminant Level Goal (MCLG) | Maximum Contaminant Level (MCL) |
|---|---|---|
| PFOA | 0 | 4.0 ppt |
| PFOS | 0 | 4.0 ppt |
| PFNA | 10 ppt | 10 ppt |
| PFHxS | 10 ppt | 10 ppt |
| HFPO-DA (GenX chemicals) | 10 ppt | 10 ppt |
| Mixture of two or more: PFNA, PFHxS, HFPO-DA and PFBS |
Hazard Indez of 1 | Hazard Indez of 1 |
Note: ppt = part per trillion (commonly used contaminant metric) Hazard Index of 1 indicates that the aggregate levels of PFAS found exceed levels where risks of health effects exist. Source: U.S. EPA (2024)
PFAS Testing Results for Oklahoma Public Water Systems
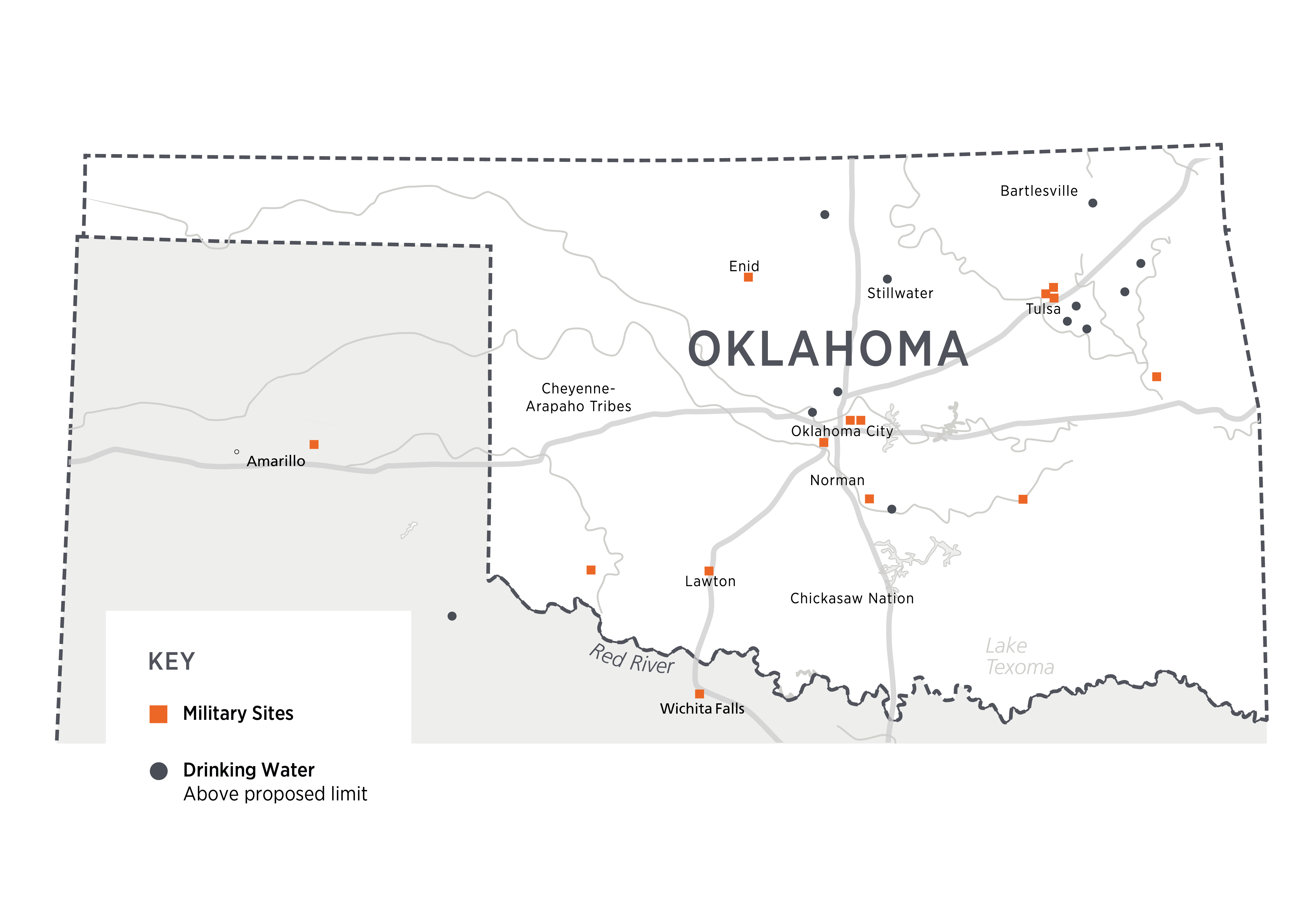
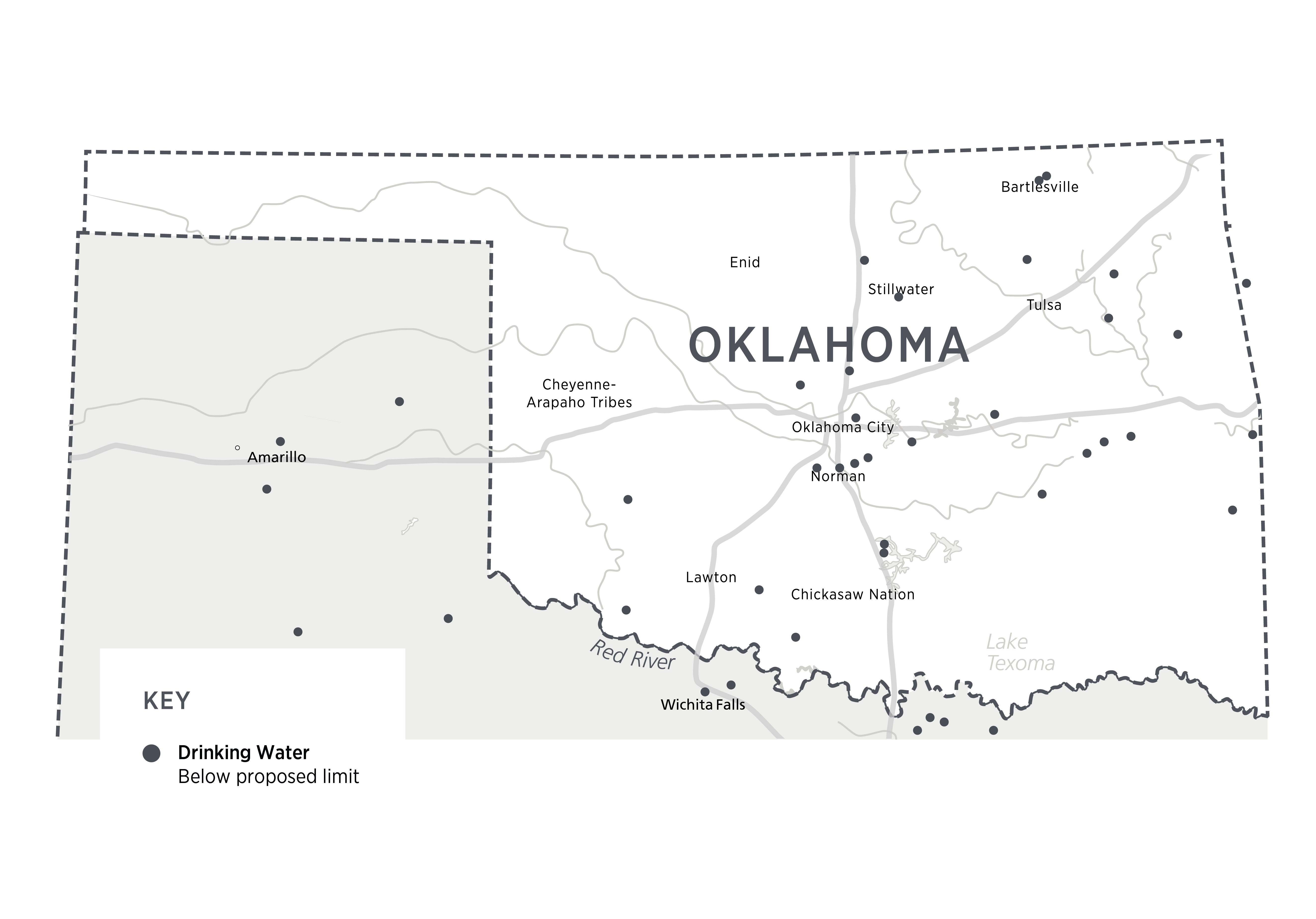
August 2024 data from the EPA has been summarized to demonstrate public water system locations across the country that have detectable levels of PFAS (EWG, 2024). The Oklahoma sites tested are displayed in Figures 1 and 2 below. An interactive site is also linked. The maps show that there are 12 military locations across the state with known PFAS contamination, and 9 public water systems where PFAS levels above EPA’s proposed limit have been detected (Figure 1). A sample report for the Stillwater Water Plant is also included. There are another 30 systems where PFAS have been detected but are not currently above the threshold set by the EPA (Figure 2). Reports for all locations are available at the interactive link.
Figure 1. Visual representation of PFAS in water (does not depict actual PFAS).
Details
System Name: Stillwater Water Plant
State: Oklahoma
Population served: 53,000
Source: EWG and EPA Unregulated contaminant Monitoring Rule
Levels listed are for the maximum of each PFAS detected at the time of the tests and do not reflect whether a water system is treating the water to reduce levels.
| Chemical | Years Tested | Maximum Level |
|---|---|---|
| PFAS Detected | ||
| PFBS | 2023 | 3.0 |
| PFHXS | 2023-2024 | 6.4 |
| PFHXA | 2023-2024 | 4.9 |
| PFOS | 2023-2024 | 8.6 |
| PFPEA | 2023-2024 | 4.4 |
Figure 2. Public Water Systems in Oklahoma with PFAS levels ABOVE proposed limits,or Military Sites with PFAS Contamination, Aug 2024.
Figure 3. Public Water Systems in Oklahoma with PFAS levels BELOW proposed limits, Aug 2024.
Impacts of the EPA PFAS Rule
Once implemented, the EPA PFAS rule is expected to help reduce the rate at which people are exposed to these chemicals. According to EPA, nearly 100 million Americans that rely on public drinking water systems will be less exposed to these chemicals via those systems. The goal of this rule is to not only improve the health of people but prolong their lifespan as well. The limits should also play a role in reducing tens of thousands of PFAS-related illnesses including cancer and liver diseases.
In-Home Treatment Option for PFAS
If you are concerned about the presence of PFAS in your water system, there are steps you can take at home to reduce potential exposure. Three recent treatment options have proven efficient in removing PFAS (especially PFOA and PFOS) from drinking water. These technologies can be used in homes at the point of entry, which is the route water uses to enter the home, or at point of use such as the kitchen sink or shower. Also, these technologies can be used in drinking water treatment facilities, water systems at hospitals or individual buildings. The following treatment options are effective at removing PFAS from drinking water when the unit is professionally installed and maintained
- High pressure membranes: This treatment option includes nanofiltration or reverse osmosis and has proven to be very efficient at removing PFAS. According to research, these membranes are typically over 90 percent efficient at removing a wide range of PFAS, including shorter chain PFAS (EPA 2018). Reverse osmosis has a more compact membrane than nanofiltration. Reverse osmosis approach uses energy to push water through tiny membrane pores. The membrane captures contaminants while allowing water to pass through. Reverse osmosis is generally considered more practical as a point-of-use treatment option (not at point-of-entry).
- Activated carbon treatment: Activated carbon is made from natural materials including wood, coal, and lignite that is high in carbon content. They are often used in a granular form called granular activated carbon (GAC). GAC has proven effective in removing PFAS from drinking water. In fact, it is 100 percent effective for a period of time, depending on the type of carbon used, the depth of the bed of carbon, water flow rate, the specific PFAS for removal, temperature, and the degree and organic matter type (EPA 2018). For example, GAC works well on longer-chain PFAS like PFOA and PFOS, but shorter chain PFAS like Perfluorobutanesulfonic acid (PFBS) and Perfluorobutyrate (PFBA) do not absorb as well. During GAC treatment, contaminants are captured in the filter while the water passes through.
- Ion exchange resin treatment: Similar to tiny powerful magnets, ion exchange resins attract and hold contaminants to it tiny beads of resin thus preventing them from passing through the water system. There are some ion exchange resin systems that may remove PFAS but make sure any system you are purchasing meets the NSF/ANSI certification standards listed below.
When buying a system for in-home PFAS treatment, look for products certified to NSF/ANSI 53 (for filters) or NSF/ANSI 58 (reverse osmosis). Note that the current certification standards for PFAS filters (as of April 2024) do not yet indicate that a filter will eliminate PFAS down to the most recent levels EPA set for a drinking water standard. EPA is working with standard-setting bodies to update their filter certifications to match EPA’s new requirements. Meanwhile, reducing PFAS levels in your water is an effective way of reducing the rate at which you are exposed to the chemical.
Rule for Privately Owned Drinking Water Systems
Many rural parts of Oklahoma depend on groundwater for daily use including watering their plants, drinking, cooking, etc. Unfortunately, EPA’s drinking water rule does not apply to drinking water from wells that serve less than 25 people including private wells that are not regulated by the Federal Government under the Safe Drinking Water Act nor by state government and laws. The quality and safety of the privately owned well is entirely with the owner of the well. However, to ensure the provision of safe drinking water in those households, EPA recommends that you do the following:
- If you are concerned about the level of PFAS in your private well/drinking water,
EPA recommends:
- Reaching out to your state environmental protection agency, health department, and/or your local utility to find out what actions are recommended.
- Use a different water source for drinking, food preparation and cooking, brushing teeth, preparing baby food, and other activities that might require your family and loved ones to swallow water.
- Think about installing an in-home water treatment (e.g., a filter) that is certified to lower the levels of PFAS in your water.
- Test private wells yearly for total coliform bacteria, nitrates, total dissolved solids, and pH levels. Also, EPA recommends using a state-certified laboratory that utilizes EPA-developed testing methods for analyses where possible.
Private well owners with little or no funds are encouraged to utilize the EPA’s grant on emerging contaminants in small or disadvantaged communities. These funds are available to assist with initial testing and treatment for both public water systems and to help private well owners address PFAS contamination issues. More information on these grants are provided later in this factsheet.
Path to Exposure
According to the Centers for Disease Control and Prevention (CDC), most Americans have already been exposed to PFAS. Recent research shows that people can be exposed to this persistent man-made chemical by:
- Drinking PFAS contaminated water
- Eating certain food that may contain PFAS, including fish
- Swallowing PFAS contaminated soil or dust
- Breathing air containing PFAS
- Using products made with PFAS or that are packaged in materials containing PFAS
- Working in areas such as firefighting or chemicals manufacturing and processing industry
How to Protect Yourself
There is no known treatment for human exposure to PFAS chemical. Therefore, it is important to have an idea of how you can protect yourself and your loved ones from being exposed. Below are several strategies for reducing exposure to PFAS.
- Avoid Teflon or polytetrafluoroethylene (PTFE) nonstick pans and kitchen utensils. Opt for stainless steel or cast iron instead. PFAS may be used in Teflon/PTFE products to keep food from sticking to cookware.
- Replace Teflon with non-Teflon or American made stainless steel, cast iron, glass, enameled or ceramic alternatives. Do not recycle, donate or sell Teflon pans or pots.
- Avoid Microwave popcorn. Microwave popcorn bags are often coated with PFAS chemicals on the inside. Try popping your popcorn the old-fashioned way on the stovetop.
- Avoid using personal care products with PTFE or fluoro ingredients in it. According to the Food and Drug Administra tion (FDA), PFAS have been intentionally added as ingredients in certain cosmetics including lotion, nail polish, shaving cream, foundation, lipstick, eye liner, eye shadow, mascara, and skin cleaners. These chemicals are added to cosmetics to condition and smooth the skin giving it a shiny look or to affect product consistency and texture. Examples of PFAS use as ingredients in cosmetics include polytetrafluoroethylene (PTFE), perfluorooctyl triethoxysilane, perfluorononyl dimethicone, perfluorodecalin, and perfluorohexane. Avoid these ingredients where possible when purchasing person al care products.
- Reduce consumption of fast food and carryout food. These foods often come in PFAS treated wrappers or containers.
- Watch out for local fish advisories in your community. Monitor Department of Environmental Quality fish consumption advisories for water bodies where you fish.
- Do your own research when shopping for clothes or outdoor gear and choose clothing that doesn’t have Gore-Tex or Teflon tags. Beware of all fabric labeled stain or water resistant, even when they don’t have a recognizable brand tag. PFAS are often used to make waterproof clothing.
- Skip stain resistant treatment on new carpets and furniture. Many of these coatings are made using PFAS chemicals. Children should not crawl or play on carpets that have been treated with PFAS-based products.
- Dust control, including mopping, wet dusting, and vacuuming are a must in the home as it helps to reduce hand to mouth transfer from PFAS contaminated household dust.
- Test your water for possible PFAS contamination. For homeowners considering this option, EPA recommends using an accredited laboratory to test water samples which use EPA approved testing methods. One such laboratory in Oklahoma is Accurate Environmental LLC.
PFAS Funding from the EPA
- Water Infrastructure Improvements for the Nation (WIIN) Grant: small, underserved, and disadvantaged communities grant program.
- Emerging Contaminants (EC) in small or disadvantaged communities grant (SDC).
- Training and Technical assistance for small systems funding.
Additional PFAS Resources
- Per- and Polyfluoroalkyl Substance (PFAS) - Final PFAS national primary drinking water regulation
- Emerging Contaminants (EC) in small or Disadvantaged Communities Grant (SDC)
- Fed center
- EPA general information
- Oklahoma Department of Environmental Quality PFAS sampling Standard Operating Procedure
- Oklahoma Cooperative Extension Service
- Trihydro Cooperation
References and Additional Information
Anderson, R. H., Long, G. C., Porter, R. C., & Anderson, J. K. (2016). Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere, 150, 678-685.
Anderko, L., & Pennea, E. (2020). Exposures to per-and polyfluoroalkyl substances (PFAS): Potential risks to reproductive and children’s health. Current Problems in Pediatric and Adolescent Health Care, 50(2), 100760.
Boston, C.M., Banacos, N. & Heiger-Bernays, W. (2019). Per-and polyfluoroalkyl substances: a national priority for safe drinking water. Public Health Reports, 134(2), 112–117.
Boyer, T., Sanders, L., Stoecker, A., Ferrell, S., & Melstrom R. (2017). Municipal Water Conservation in Oklahoma: Background, Issues, and Options. Oklahoma Cooperative Extension Fact Sheet AGEC-1055. Available online: extension.okstate.edu/fact-sheets/municipal-water-conservation-in-oklahoma-background-issues-and-options.html
Brennan, N. M., Evans, A. T., Fritz, M. K., Peak, S. A., & von Holst, H. E. (2021). Trends in the Regulation of Per-and Polyfluoroal kyl Substances (PFAS): A Scoping Review. International journal of environmental research and public health, 18(20), 10900.
Buck, R. (2015). Toxicology Data for Alternative “Short-Chain” Fluorinated Substances, Molecular and Integrative Toxicology, pp. 451-477.
Cordner, A., De La Rosa, V.Y., Schaider, L.A., Rudel, R.A., Richter, L. & Brown, P. (2019). Guideline levels for PFOA and PFOS in drinking water: the role of scientific uncertainty, risk assessment decisions, and social factors. Journal of Exposure Science & Environmental Epidemiology, 29, 157–171.
Danish, E. P. A. (2018). Risk assessment of fluorinated substances in cosmetic products. Survey of chemical substances in consumer products, (169).
Domingo, J. L., & Nadal, M. (2019). Human exposure to per-and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environmental research, 177, 108648.
Environmental Working Group (EWG). (2024). Interactive Map: PFAS Contamination Crisis: New Data Show 7,457 Sites in 50 States (ewg.org) Available online: ewg.org/interactive-maps/pfas_contamination/
Environmental Research & Education Foundation
erefdn.org/per-and-polyfluoroalkyl-substances-pfas-resource/
Evans, S., Andrews, D., Stoiber, T., & Naidenko, O. (2020). PFAS contamination of drinking water far more prevalent than previ ously reported. Environmental Working Group, 22.
Food and Drug Administration (FDA). (2024) Testing Food for PFAS and Assessing Dietary
Exposure. Available online:
fda.gov/food/chemical-contaminants-food/testing-food-pfas-and-assessing-dietary-exposure
Food and Drug Administration (FDA). (2024) Per and Polyfluoroalkyl Substances (PFAS) in Cosmetics. Available online fda.gov/cosmetics/cosmetic-ingredients/and-polyfluoroalkyl-substances-pfas-cosmetics
Kabadi, S., Fisher, J., Aungst, J., and Rice, P. (2018). Internal exposure-based pharmacokinetic evaluation of potential for biop ersistence of 6:2 fluorotelomer alcohol (FTOH) and its metabolites, Food and Chemical Toxicology, vol. 112, pp. 375-382
Macheka, L. R., Abafe, O. A., Mugivhisa, L. L., & Olowoyo, J. O. (2022). Occurrence and infant exposure assessment of per and polyfluoroalkyl substances in breast milk from South Africa. Chemosphere, 288, 132601.
National Toxicology Program (NTP). (2016). Monograph on Immunotoxicity Associated With Exposure to Perfluorooctanoic acid (PFOA) and Perfluorooctane Sulfonate (PFOS). Research Triangle Park, NC: National Toxicology Program; ntp.niehs.nih.gov/ntp/ohat/pfoa_pfos/pfoa_pfosmonograph_508.pdf
National Institute of Environmental Health Sciences (NIEHS). Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). niehs.nih.gov/health/materials/perfluoroalkyl_and_polyfluoroalkyl_substances_508.pdf
Oklahoma Water Resource Board (OWRB). (2024). Fact Sheet. Available online: https://www.owrb.ok.gov/util/waterfact.php
Steenland, K., Fletcher, T., Stein, C. R., Bartell, S. M., Darrow, L., Lopez-Espinosa, M. J., & Savitz, D. A. (2020). Evolution of evi dence on PFOA and health following the assessments of the C8 Science Panel. Environment International, 145, 106125.
U.S. Environmental Protection Agency (EPA). (2017). Factsheet Technical Fact Sheet – Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) Available online: 19january2021snapshot.epa.gov/sites/static/files/2017-12/documents/ffrrofactsheet_contaminants_pfos_pfoa_11-20-17_508_0.pdf
U.S. Environmental Protection Agency (EPA). EPA Fact Sheet on PFAS Regulation: epa.gov/system/files/documents/2024-04/pfas-npdwr_fact-sheet_general_4.9.24v1.pdf
U.S. Environmental Protection Agency (EPA). (2024). Private Drinking Water Wells. epa.gov/privatewells
U.S. Environmental Protection Agency (EPA). (2024). Understanding the Final PFAS National Primary Drinking Water Regu lation Hazard Index Maximum Contaminant Level. Available online: epa.gov/system/files/documents/m2024-04/pfas-npdwr_fact-sheet_hazard-index_4.8.24.pdf
Wang, Z., Cousins, I., Scheringer, M., and Hungerbuhler, K. (2013). Fluorinated alternatives to long-chain perfluoroalkyl carbox ylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors, Environmental International, vol. 60, pp. 242-248.
Wei, Z., Xu, T., & Zhao, D. (2019). Treatment of per-and polyfluoroalkyl substances in landfill leachate: status, chemistry and prospects. Environmental Science: Water Research & Technology, 5(11), 1814-1835.
Xu, B., Liu, S., Zhou, J. L., Zheng, C., Weifeng, J., Chen, B., & Qiu, W. (2021). PFAS and their substitutes in groundwater: Occur rence, transformation and remediation. Journal of hazardous materials, 412, 125159.
Zheng, G., Schreder, E., Dempsey, J. C., Uding, N., Chu, V., Andres, G., . & Salamova, A. (2021). Per-and polyfluoroalkyl sub stances (PFAS) in breast milk: Concerning trends for current-use PFAS. Environmental Science & Technology, 55(11), 7510-7520.