Lipid Glossary
This fact sheet covers lipid-related terminology commonly used in industry and the scientific literature. See Figure 1 and Table 1 for fatty acid chemical structure and naming systems, respectively.
Table 1. Fatty Acid Naming Systems
| Common Name | International Union of Chemistry (IUC) Name | Numeric Abbreviation (Number of carbon atoms: number of double bonds followed by the position of the double bond on the fatty acid carbon chain) | Numeric Abbreviation (Number of carbon atoms: number of double bonds followed by the position of the double bond on the fatty acid carbon chain) |
|---|---|---|---|
| Delta (Δ)* | |||
| palmitic acid | hexadecanoic acid | 16:0 | 16:0 |
| stearic acid | octadecanoic acid | 18:0 | 18:0 |
| oleic acid | 9-octadecanoic acid | 18:1 Δ9 | 18:1 (ω-g) |
| linoleic acid | 9, 12-octadecenoic acid | 18:2 Δ9, 12 | 18:2 (ω-6) |
| linolenic acid | 9, 12, 15-octadecenoic acid | 18:3 Δ9, 12, 15 | 18:3 (ω-3) |
Acylglycerol: A systematic name for all types of fatty acids esterified to a glycerol molecule. Examples include mono, di- and triacylglycerols.
Amphiphilic: A molecule with a polar head group and one or more lipophilic carbon tails. Surfactants and emulsifiers are good examples for this group of compounds.
Behenic acid: Trivial name for the saturated fatty acid docosanoic acid (C22:0).
Brassica sterol: A phytosterol (plant sterol) mainly present in rape, canola and mustard seed oils. Some other seed oils may contain brassica sterol at much lower concentrations.
Butyric acid: It also is referred to as butanoic or tetranoic acid (C4:0), which is a short-chain saturated fatty acid.
Campesterol: One of the phytosterols commonly found in plants and oilseeds.
Capric acid: Trivial name for the saturated fatty acid decanoic acid (C10:0).
Caproic acid: Trivial name for the saturated fatty acid hexanoic acid (C6:0).
Caproleic acid: Trivial name for the monounsaturated fatty acid 9-decenoic acid (C10:1).
Caprylic acid: Trivial name for the fatty acid octanoic acid (C8:0).
Ceramide: Trivial name for the lipid class N-acylsphingosines, the building block of the complex sphingolipids. They are widely used in cosmetics.
Cholesterol: The most common animal sterol. It may occur in free or esterified form. Cholesterol concentration in vegetable oils is very low, maximum 10-20 mg/kg oil. Animal fats contain higher amounts of cholesterol, i.e. 3700-4200 mg/kg in lard, 2300-3100 mg/kg in mutton tallow, 800-1400 mg/kg in beef, and about 300 mg of cholesterol per egg or about 5 percent of total lipids in hen eggs.
Decanoic acid: Systematic name for the saturated fatty acid caproic acid (C10:0). It occurs in coconut and palm kernel oils and is a minor component of milk fat.
Diacylglyceride (DAG): An acylglyceride with two fatty acid molecules esterified to the hydroxyl groups on a glycerol molecule. DAGs are widely used as emulsifiers in food formulations.
Docosahexaenoic acid (DHA): It is a long-chain highly unsaturated (22:6) omega-3 (n-3) fatty acid. The trivial name is cervonic acid. This acid is abundant in fish oils and a significant component of membrane lipids of most animal tissues especially lipids of brain, sperm and the retina of the eye.
Docosanoic acid: A saturated (C22:0) fatty acid. Its trivial name is behenic acid.
Dodecanoic acid: A major saturated fatty acid (C12:0) in lauric oils and oils of the Cuphea family. The trivial name is lauric acid.
Eicosanoic acid: A saturated (C20:0) fatty acid. The trivial name is arachidic acid.
Eicosapentaenoic acid (EPA): A highly unsaturated long-chain n–3 fatty acid (20:5). This acid is present in most fish and algal oils.
Eicosatetraenoic acid: There are several isomers of this long-chain unsaturated fatty acid (C20:4). The n-6 all cis isomer (5cis, 8cis, 11cis, 14cis) of this fatty acid is known as arachidonic acid. It is present in fish oil in minor amounts, mostly associated with animal phospholipids and also present in some ferns. It is commercially produced from liver or egg lipids and by fermentation.
Eicosenoic acid: It is a long-chain monounsaturated fatty acid (C20:1). The trivial names for 9cis and 11cis isomers are gadoleic and gondoic acid, respectively.
Elaidic acid: The trans isomer of oleic acid (C18:1) and trivial name for 9trans, octadecenoic acid.
Elaido acids: The term elaido often is used to distinguish trans isomers from the cis forms, i.e. linelaidic (9trans, 12trans octadecadienoic acid) and linolenelaidic (9trans, 12trans, 15trans, octadecatrienoic acid).
Epoxy acids: These fatty acids are produced by epoxidation of olefinic acids. Vernonia galamensis and Euphorbia lagascae seeds are rich in vernolic acid, which is an epoxy fatty acid.
Erucic acid: Trivial name for the monounsaturated fatty acid docosenoic acid (C22:1, 13cis). It is present in seed oils of the Cruciferaefamily such as rape, mustard and crambe. Modern varieties of rapeseed oils have been bred to contain less than 2 percent erucic acid, down from 30-50 percent because of the adverse health effects of this fatty acid. Oils with high erucic acid content are used in oleochemical industry.
Essential fatty acids: Polyunsaturated acids (linoleic and linolenic acids), which are essential for life and good health. They cannot be biosynthesized by animals and humans, so they must be obtained through diet.
FAME: Abbreviation for methyl esters of fatty acids that are mostly used for gas chromatography.
Fats: Fats are a subgroup of lipids and comprised of mostly triacylglycerides. They are the bulk storage lipids produced by animals and microorganisms. They are solid or semisolid at room temperature.
Fatty Acids: Fatty acids are saturated or unsaturated carboxylic acids with a long straight and unbranched carbon chain (Figure 1). Fatty acids are the major components of most lipids. They may occur in free or esterified (attached to another molecule through an oxygen bond) forms. The majority of the fatty acids in oils and fats are esterified to glycerol molecules forming triacylglycerides, diacylglycerides, monoacylglyceride and phospholipids. Table 1 summarizes fatty acid naming systems.
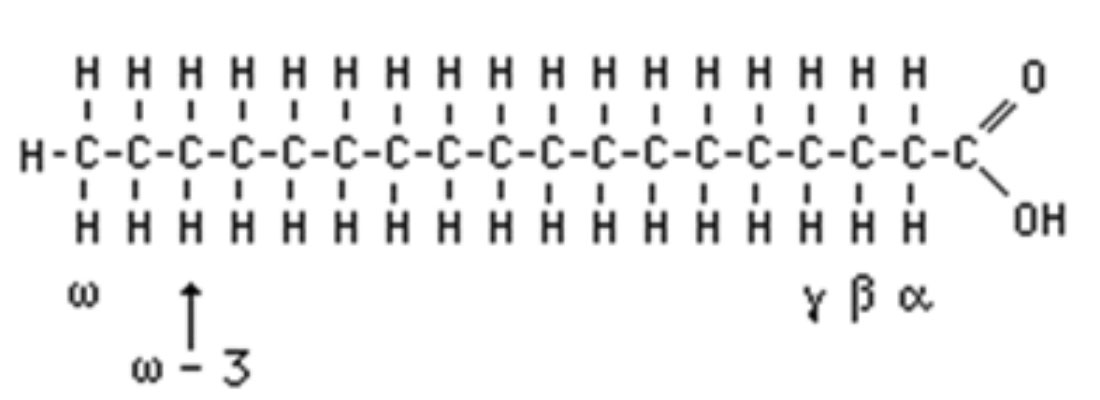
Figure 1. Chemical structure of a fatty acid molecule
There are different fatty acid naming systems. Trivial names contain no clues to the structures; one must learn the name and associate it with a separately learned structure. The names typically derive from a common source of the compound or the source from which it was first isolated. For example, palmitic acid is found in palm oil, oleic acid is a major constituent of olive oil (oleum) and stearic (from the Greek word meaning solid) acid is solid at room temperature. Spiders (arachnids) contain arachidonic acid.
The carboxyl reference system or numeric abbreviation indicates the number of carbons, the number of double bonds and the positions of the double bonds, counting from the carboxyl carbon, which is numbered as one. The fatty acid naming convention developed by the International Union of Chemistry (IUC) is based on Latin names for the carbon chain length and saturation or unsaturation (double bonds) on the molecule. Carboxyl reference differs from the IUC nomenclature in that it uses a number to denote chain length instead of a name derived from Greek (e.g., hexadecanoic acid for 16:0 or palmitic acid). In the IUC system, the carboxyl carbon is denoted by the number one (Figure 1), and positions in the chain are denoted with reference to it. For example a double bond is said to be at the ninth carbon if it originates at the ninth carbon and extends to the next (10th) carbon in the chain. The position is denoted by “Δ” (see Table 1).
The omega (ω or n) reference system indicates the number of carbons, the number of double bonds and the position of the double bond closest to the omega carbon, counting from the omega carbon (which is numbered one for this purpose) (Figure 1). This system is useful in physiological considerations because of the important physiological differences between omega negative three and omega negative six fatty acids in the human body.
Free fatty acids (FFA): These are the fatty acids in unbound form (not esterified). Crude oils and fats contain FFA, which may be removed by processing, chemical neutralization or physical refining (see Fact Sheet FAPC-160). FFA have important physiological functions in animal and plant tissues but not desirable in edible oils because they lower the oxidative stability and quality of the oils.
Gadelaidic acid: Trivial name for the monounsaturated fatty acid 9trans eicosenoic acid (20:1).
Gadoleic acid: Trivial name for the monounsaturated fatty acid 9cis eicosenoic acid (20:1) present in fish oil.
Gaidic acid: Trivial name for the monounsaturated fatty acid 2-hexadecenoic acid (16:1).
Galactolipids: The lipids that contain one or more galactose units. Examples include mono and digalactosyldiacylglycerols, glycosylceramide and galactosylglycerides.
Galactosylceramide: It is a galactolipid, also referred to as glycosylceramide.
Galactosylglycerides: Diacylglycerols with one to four (commonly one or two) galactose units attached to the sn-3 position of a triacylyglyceride (see Figure 2 for sn-3 position description) molecule by glycosidic bonds. They are common membrane lipid components of plants (chloroplasts).
Gamma linolenic acid (GLA): Common name for the polyunsaturated fatty acid octadecatrienoic acid (C18:3), γ-linolenic acid.
Glucosinolates: Sulphur containing compounds present in rape seed. They have been reduced to less than 30 mg/kg seed in canola and the double-zero varieties through biotechnology.
Glycerol: Trivial name for 1,2,3-trihydroxypropane. The most common natural carrier for acyl groups and the basis of many lipid classes such as triacylglycerols and glycerophospholipids.
Glycerophospholipids: These are lipids containing a phosphate ester at the sn-3 position and acyl groups in the sn-1 and sn-2 positions of a glycerol molecule (see Figure 2 for sn position numbering). The main classes are phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidylinositol, phosphatidylserine and diphosphatidylglycerol.
Glycolipids: General name for all lipids linked to any type of carbohydrate moiety. The main plant glycolipids are mono and digalactosyldiacylglycerols. Sterol glycosides and cerebrosides also are present in lesser amounts. Glycosphingolipids such as gangliosides and cerebrosides are mainly present in animals.
Gondoic acid: Trivial name for the monounsaturated fatty acid eicosenoic acids (C20:1).
Gossypol: A complex phenolic compound occurring in crude cottonseed oil and removed during the refining process.
Heptadecanoic acid: Trivial names for this saturated fatty acid, heptadecanoic acid (C17:0), are daturic and margaric acid. This fatty acid is present in many animal, especially in ruminants and in some vegetable oils in very low amounts.
Hexacosanoic acid: The trivial name for this saturated fatty acid, hexacosanoic acid (C26:0), is cerotic acid.
Hexadecanoic acid: The trivial name for hexadecanoic acid (C16:0) is palmitic acid, which is a saturated fatty acid. It is the most common of all saturated fatty acids. It is present in virtually all animal and vegetable fats, especially in palm oil (about 40 percent) and cottonseed oil (about 25 percent). In vegetable oils this acid occurs in the sn-1 and sn-3 positions and hardly at all in the sn-2 positions of a TAG molecule. Lard and most milk fats, including human milk fat, have unusually high levels of hexadecanoic acid in the sn-2 position.
Hexadecenoic acids: Palmitoleic and zoomaric acids are trivial names for 9cis hexadecenoic acid (C16:1), which is a monounsaturated fatty acid. It is present in most fish oils (about 10 percent) and macadamia oil (about 20 percent).
Hexanoic acid: The trivial name for the saturated fatty acids hexanoic acid (C6:0) is caproic acid. It is present at low levels in lauric oils and milk fat.
Hydroxy acids: These fatty acids contain a hydroxyl group in their chemical structure. Ricinoleic acid is the best known member of this class. Others include aleuritic, cerebronic, coriolic, densipolic, dimorphecolic, kamlolenic and lesquerolic acid. ω-Hydroxy acids (C14-C22) occur in waxes.
Interesterification: It refers to the production of esters by interaction of two esters in the presence of an alkaline or enzymatic catalyst. Interesterification of oils and fats leads to randomization of the fatty acids on triacylglycerol molecules and produces oils or fats with different characteristics than the oils or fats used in the process.
Kephalin: It also is referred to as cephalin. Kephalin is the old term used for phosphatidylethanolamine.
Lauric acid: Trivial name for the saturated fatty acid dodecanoic acid (C12:0), found mainly in lauric oils such as coconut and palm kernel.
Lecithin: It refers to a crude mixture of phospholipids obtained from vegetable oils and egg yolk. Most lecithin is derived from soybean oil and is recovered during the degumming process. Typically, lecithin is a mixture of phospholipids (47 percent), triacylglycerols (36 percent), other lipids (11 percent), carbohydrates (5 percent) and water (1 percent). Phosphatidylcholine, phosphatidylethanolamine and phosphatidylinositide are the main phospholipids present in lecithin.
Lesquerolic acid: This is a hydroxy fatty acid (contains two hydroxyl group structure), one double bond and 20 carbons on the chemical structure. It is present in seed oils of the Lesquerella species.
Lignoceric acid: Trivial name for the saturated fatty acid tetracosanoic acid (C24:0). It occurs in some waxes and is a minor component of some seed oils.
Linelaidic acid: Trivial name for the polyunsaturated fatty acid 9trans, 12trans-octadecadienoic acid (C18:2). It rarely occurs in nature.
Linoleic acid: Trivial name for the polyunsaturated fatty acid 9cis, 12cis octadecadienoic acid (C18:2).
Linolenic acid: Trivial name for the polyunsaturated fatty acid octadecatrienoic acid (C18:3). It exists in two isomeric methylene-interrupted forms: α-linolenic acid (n–3) and γ-linolenic acid (n–6).
Lysophospholipids: Phospholipids containing only one acyl chain are referred to as lysophospholipids. Depending on the phosphate group attached to the molecule, they are further classified as lysophosphatidylcholine and lysophosphatidylethanolamine, etc.
Margaric acid: Trivial name for the saturated fatty acid heptadecanoic acid (C17:0).
Medium-chain triacylglycerols: Oils commercially prepared by esterification of glycerol with octanoic (C8) and decanoic (C10) acids obtained from coconut and palm kernel oils. These esters are easily digested and are used for nutritional and pharmaceutical purposes. They are liquids with high oxidative stability and also find use as food-grade lubricants.
Miscella: Mixture of hexane and crude oil produced during solvent extraction of vegetable oils.
Micelle: Small colloidal aggregate formed in water by soluble amphiphiles (surfactants).
Monoacylglyceride (MAG): An acylglyceride with only one fatty acid molecule esterified to one of the hydroxyl groups on a glycerol molecule. MAGs are used as emulsifiers in bakery products, beverages, ice cream, chewing gum, shortening, whipped toppings, margarine and confections. They can be produced from TAG or DAG through chemical or enzymatic processes.
Myristic acid: Trivial name for the saturated fatty acid tetradecanoic acid (C14:0). This acid is present in coconut oil and palm kernel oil in significant amounts, 15-20 percent of the total fatty acids and is a minor component of most animal fats and fish oils.
Nervonic acid: Trivial name for the monounsaturated fatty acid 15cis tetracosenoic acid (C24:1).
Neutral lipids: These are non-polar lipids in contrast to polar lipids. Triacylglycerols, sterols, and sterol esters are some of the examples of neutral lipids.
Octadecadienoic acids: The most important octadecadienoic acid (C18:2) is linoleic acid (9cis 12cis isomer), which is present in virtually all seed oils. It is the first member of the n-6 family of polyene acids and is an important essential fatty acid.
Octadecanoic acid: It is the saturated fatty acid (C18:0) that is commonly known as stearic acid. This is the second most common saturated acid after hexadecanoic (palmitic) acid. It is a minor component of most vegetable oils but is present in larger amounts in cocoa butter (30-36 percent) and in ruminant fats (5-40 percent).
Octadecatetraenoic acid: It is a highly unsaturated fatty acid (C18:4). The 6cis, 9cis, 12cis, 15cis isomer is known as Stearidonic acid and a member of the n-3 family. The 4cis, 8cis, 12cis, 15cis isomer is referred to as moroctic or morotic acid.
Octadecatrienoic acids: The most common and important octadecatrienoic acid (C18:3) is α-linolenic acid (9cis, 12cis, 15cis isomer). This is a major component in drying oils such as linseed oil, 50-60 percent of the total fatty acids. It is a member of the n–3 family and is an essential fatty acid. The 5cis, 9cis, 12cis isomer is known as pinolenic acid and present in tall oil and in seed oils of many conifer species.
Octadecenoic acids: The general name for any C18:1 acid including oleic, elaidic, vaccenic, petroselinic and petroselaidic.
Octanoic acid: It is an 8-carbon saturated fatty acid (C8:0) also known as caprylic acid. A medium-chain fatty acid occurring in the lauric oils and in Cuphea seed oils.
Oil: Oils are a subgroup of lipids and comprised of mostly triacylglycerides. They are liquid at room temperature and mainly originate from plants.
Oil bodies: Also referred to as oleosomes, oleosins or lipid bodies, they are cellular organelles in oil seed cells and contain mainly triacylglycerols.
Oleic acid: Trivial name for 9cis isomer of octadecenoic acid (C18:1), which is a monounsaturated fatty acid.
Olein: Liquid fraction produced by fractionation of oils or fats. It is most commonly produced from palm oil.
Oleochemicals: These are the compounds produced from natural oils and fats and include fatty acids, alcohols, methyl or other esters, amides and amines, dimer acids and dibasic acids, and are the basis of surfactants and other compounds.
Omega-3 Fatty Acids: They are abbreviated as n-3 or ω-3 (omega-3) acids. In general these polyunsaturated fatty acids have three or more cis unsaturated bonds separated from each other by one methylene group and having the first unsaturated on the third carbon from the methyl end of the molecule (Figure 1). α-Linolenic acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are some examples for omega-3 fatty acids (see Fact Sheet FAPC-135).
Omega-6 Fatty Acids: They are abbreviated as n-6 or ω-6 (omega-6) acids. A family of polyunsaturated fatty acids with two or more cisunsaturated bonds separated from each other by one methylene group and having the first unsaturated bond on the sixth carbon from the methyl end of the molecule. Arachidonic acid (eicosatetraenoic acid) is an example for an omega-6 fatty acid.
Omega-9 Fatty Acids: They are abbreviated as n-9 or ω-9 (omega-9) acids. A family of polyunsaturated fatty acids with two or more cisunsaturated bonds separated from each other by one methylene group and having the first unsaturated bond on the ninth carbon from the methyl end of the molecule. Gondoic (20:1), erucic (22:1), nervonic (24:1), ximenic (26:1), octacosenoic (28:1) and lumequic acids (30:1) are some of the examples of omega-9 fatty acids.
Palm fatty acid distillate: It is a byproduct of the physical refining of palm oil rich in free acids. Their calcium salts are used in animal feed formulations.
Palmitelaidic acid: Trivial name for the 9trans isomer of hexadecenoic acid (C16:1).
Palmitic acid: Trivial name for the saturated fatty acid hexadecanoic acid (C16:0).
Palmitoleic acid: The 9cis isomer of hexadecenoic acid (C16:1), also known as zoomaric acid.
Palm mid fraction: It is produced by fractionation of palm oil. This product is commonly used as a cocoa butter equivalent.
Palm olein: It is produced by fractionation of palm oil and liquid at room temperature. It is used as a stable frying oil.
Palm stearin: Solid fraction obtained by fractionating palm oil. It is used as a hard fat in margarine stock or as an alternative to tallow in the oleochemical industry.
Phospholipid: Phospholipids are esters of glycerol containing two fatty acids and a phosphatidic acid moiety on the sn-3 position (see Figure 2).
Phytosterols: Phytosterols are plant-derived sterols. The most important phytosterols in soybean are campesterol, stigmasterol and sitosterol.
Polar lipids: Lipids with polar groups as the head group, e.g. phospholipids and glycolipids.
PUFA: Polyunsaturated fatty acids that have two or more double bonds on the carbon chain.
Reversed micelles: They are also called microemulsions; water aggregates in an oil phase.
Rhamnolipids: Glycolipids containing the sugar rhamnose and 3-hydroxy carboxylic acids.
Saponification: Alkaline hydrolysis of oils and fats yielding glycerol and sodium or potassium salts of the long-chain acids.
Saturated acids: Fatty acids without carbon-carbon bond unsaturation (no double bond on the carbon chain). Examples include lauric (12:0), myristic (14:0), palmitic (16:0) and stearic (18:0) acids.
Shortening: The common name for solid fat used in baking and cooking.
Specialty oils: Oils that produced in relatively small quantities for their distinctive properties such as flavor and health benefits.
Stearic acid: Trivial name for the saturated fatty acid octadecanoic acid (18:0).
Stearidonic acid: Trivial name for the polyunsaturated fatty acid 6cis, 9cis, 12cis, 15cis-octadecatetraenoic acid (C18:4).
Stearin: Less-soluble higher-melting fraction of oils and fats that has a higher content of saturated fatty acids.
Structured fats: Produced to have a particular property such as lower energy value, a specific melting behavior or nutritional property.
Surfactants: Surface-active compounds that contain lipophilic (hydrophobic) and hydrophilic (lipophobic) sections within the molecules. They are used as detergents, emulsifiers, flotation agents, etc.
Tallow: Animal edible tallow, normally obtained from beef but also from sheep and goats.
Tetracosenoic acid: Monounsaturated fatty acid that also is called nervonic or selacholeic acid (24:1, 15cis).
Tetradecanoic acid: Saturated fatty acid that also is called myristic (C14:0).
Tetradecenoic acid: Medium-chain monounsaturated fatty acid that also is called myristoleic acid (C14:1, 9cis-tetradecenoic acid).
Trans fatty acids: These fatty acids have trans (E) unsaturated centers, in contrast to the cis (Z) configuration in most natural acids. Transfatty acids in edible oils mainly produced during the partial hydrogenation of vegetable or fish oils. They are present in very small amounts in seed oils. Fats from ruminal animals contain small amount of trans fat as well (see Fact Sheets FAPC-133 and FAPC-134).
Transesterification: A technique that produces esters from other esters by acid, base or enzymatic catalysis. Newly formed ester have different properties than the starting material. This technique is used for modification of fats and oils and production of biodiesel.
Triacylglyceride (TAG): A TAG is an ester derived from a glycerol and three fatty acid molecules. They are the main constituents of fats and oils and principal lipids in the blood. A schematic of a TGA molecule is shown in Figure 2. TAG is a neutral molecule (no electric charge on the molecule). It is the main storage form of lipids in animals and plants.
Unsaturated fatty acids: Fatty acids with one or more unsaturated centers, or carbon-carbon double bonds.
Vaccenic acid: Trivial name for the monounsaturated fatty acid (C18:1) 11-octadecenoic acid (usually the trans isomer).
Valeric acid: Trivial name for the short chain saturated fatty acid pentanoic acid (C5:0).
Waxes: Water-resistant materials made up of hydrocarbons, long-chain acids and alcohols, wax esters and other long-chain compounds. Examples include both animal (beeswax, wool wax (lanolin), sperm whale wax) and plant (candelilla, carnauba, rice-bran, sugar cane and jojoba) based waxes.
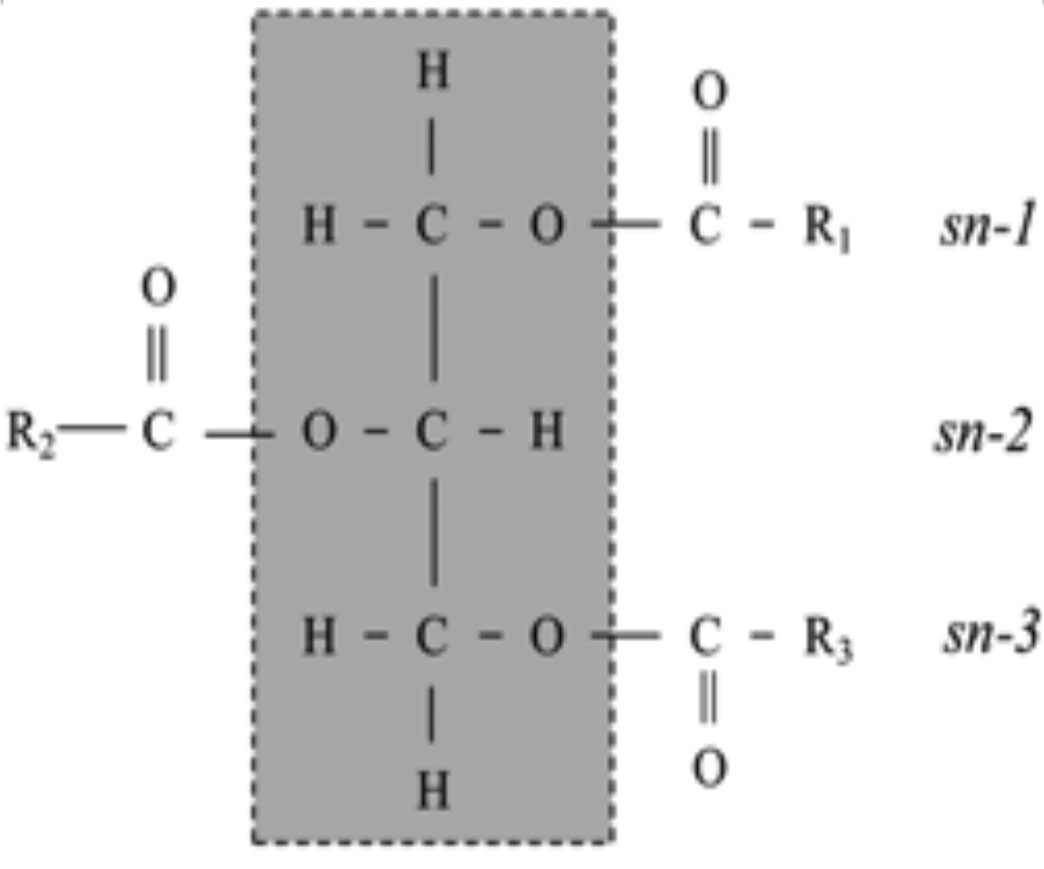
Figure 2. Schematic of a triacylglyceride molecule structure. R1, R2, and R3 indicating the fatty acid and the sn-1, -2 and -3 positions
Nurhan Turgut Dunford
FAPC Oil/oilseed Specialist
