Common Diseases of Tomatoes- Part II: Diseases Caused by Bacteria, Viruses and Nematodes
Diseases of tomato caused by bacteria, viruses and nematodes can be severe, reduce tomato yield and quality and generally are more difficult to control than those caused by fungi. Management of these diseases is most effective with the integrated use of practices such as crop rotation, resistant varieties, sanitation and disease exclusion. The intent of this fact sheet is to aid tomato growers in the identification of diseases caused by bacteria, viruses and nematodes, and to provide guidelines for their management. Chemical control strategies that may be required are updated annually in the OSU Extension Agents’ Handbook of Insect, Plant Disease and Weed Control (Circular E-832).
Bacterial Diseases
Bacteria are microscopic, single-celled organisms. Bacteria multiply rapidly by simple cell division and absorb nutrients from their immediate environment. Bacterial pathogens are introduced to new sites on contaminated seed or transplants. Once established, bacteria are spread by splashing rain, water runoff, wind-driven rain or mists (aerosols), equipment, insects and people working around the plants. Bacteria persist in and around tomato plantings in weeds, volunteer plants, infested crop debris and as symptomless colonizers of plant surfaces.
Bacterial spot
(Xanthomonas euvesicatoria)
Bacterial spot is a widespread disease of tomato in Oklahoma, and can be particularly severe in eastern Oklahoma, where rainfall and humidity levels promote disease development. Bacterial spot reduces tomato yield and quality by defoliation and spotting of fruit. Bacterial spot is also a serious disease of pepper. The bacteria survive on diseased plant debris and on tomato seed. Leaves, stems and fruit may be infected at any growth stage when plants are wet and temperatures range from 75 F to 86 F. The bacteria enter plants through natural openings or wounds. Leaf spots are dark brown in color, appear greasy when leaves are wet and rarely exceed 1/8 inch in diameter (Figure 1). The leaf spots produced in bacterial spot are nearly identical to those of bacterial speck, and are similar to the initial symptoms of the fungal disease Septoria leaf spot. Bacterial spots lack the grey centers typical within older leaf spots caused by Septoria. A general yellowing of heavily spotted areas on leaves occurs, followed by leaf scorch. Blighting (rapid death) of foliage progresses upward from the lower leaves on heavily infected plants. Fruit spots are conspicuous on green fruit and appear raised and scabby, dark brown in color, and are up to 1/3 inch in diameter (Figure 2). Spots on ripe fruit are similar except they are sunken.
Control of bacterial spot is difficult once it becomes established in the field. Therefore, it is imperative to start with clean seed and transplants. Transplants should be started from clean seed produced in an area where the disease does not occur, or from seed that is disinfested with hot water or chlorine bleach treatment. Hot-water treatment involves soaking seed at exactly 122 F for 25 minutes to 30 minutes, followed by cooling, drying and treatment with a fungicide for control of seed rot and damping off. However, germination of weak seed (more than one year old), pepper seed or excessively heated seed may be reduced by this treatment. The chlorine treatment involves soaking seed in a solution of one part household bleach (5.25 percent sodium hypochlorite) and two parts water for one minute followed by rinsing, drying and treatment with a fungicide. Remove and destroy diseased crop debris or incorporate it into soil soon after harvest is complete. Rotate tomatoes with crops other than tomato and pepper to avoid carryover of bacteria from year to year. Using drip irrigation reduces bacterial spread and the leaf wetness periods that favor infection compared to sprinkler irrigation. Avoid working in plantings when foliage is wet. A weekly spray program with bactericides (mixtures of copper and fungicide) reduces disease development and increases yield.

Figure 1. Bacterial spot – leaf spots.

Figure 2. Bacterial spot – fruit spots.
Bacterial speck
(Pseudomonas syringae pv. tomato)Bacterial speck is a disease of increasing importance. Yield loss results from defoliation and the reduced market value of the specked fruit. The disease affects both leaves and fruit. While leaf wetness is required for both bacterial spot and bacterial speck infection, cooler temperatures (55 F to 77 F) favor speck. Spots on leaves cannot be easily distinguished from those of the bacterial spot disease. Leaf spots are small, dark brown to black in color, and may be surrounded by a yellow border (Figure 3). Only green fruit are susceptible to infection. Fruit symptoms are more characteristic, appearing on green fruit as dark, superficial specks usually less than 1/16 inch in diameter (Figure 4). The areas surrounding spots may be a more intense green color. Specks may be raised on green fruit, but become sunken on ripe fruit with a zone of delayed ripening immediately surrounding the speck. Management practices for this disease are the same as for bacterial spot.

Figure 3. Bacterial speck – leaf spots.

Bacterial canker
(Corynebacterium michiganensis)
Bacterial canker is a sporadic, but damaging disease of tomato in Oklahoma. Bacterial canker can be difficult to diagnose because a variety of symptoms may occur and the canker symptom (stem lesion) is not always produced. Plants affected early in their development wilt and die and the disease may be confused with bacterial wilt. Infected plants develop a leaf scorch (Figure 5) and begin dying from the bottom up. Splitting the stems of affected plants reveals streaks of yellow to reddish brown internal discoloration. Eventually, the pith becomes reddish brown in color and mealy in texture. Whitish cankers on stems and leaf petioles in the upper portion of affected plants may develop with certain conditions. The “bird’s- eye” spots that often develop on fruit can be useful in diagnosing the disease because of their distinct appearance. Fruit spots are small (1/8 inch to 1/4 inch in diameter), with raised brown centers surrounded by a white halo (Figure 6). Plants that are infected early are killed, while those that become infected later from spread of the bacterium within a field may only develop leaf scorch and fruit symptoms.
The bacterium is carried on seed and on transplants, and can survive for short periods in soil, contaminated greenhouse structures and wooden tomato stakes. The bacterium survives for longer periods in plant debris and may cycle on nightshade weeds and volunteer tomato plants. In the field, the bacterium is spread by splashing water and wind-driven rain; contaminated equipment; and by pruning, staking and harvesting activities.
Clean seed and transplants are important for preventing the introduction of the pathogen into greenhouses and fields. Assume all seed is contaminated, and apply either the hot water or chlorine bleach seed treatments described in the bacterial spot section. If tomato stakes from a problem field are to be reused, they should be soaked in a 1 percent bleach solution. Infected plants in the field should be incorporated into the soil soon after harvest is complete to hasten decomposition of the debris. Rotation of tomatoes with crops other than tomato or pepper is encouraged. Spray programs with bactericides have not been effective against this disease.

Figure 5. Bacterial canker – leaf scorch.

Figure 6. Bacterial canker – “bird’s eye” spots on fruit.
Bacterial wilt
(Ralstonia solanacearum)
Bacterial wilt is a devastating disease of tomato, tobacco and potato in southern states. The bacterium survives freely in soil for extended periods of time and infection occurs through roots. Symptoms are rapid wilting and death of plants without yellowing or spotting of leaves (Figure 7). Brown discoloration and decay are evident inside the stems of infected plants. The disease is easily diagnosed by suspending a clean, cut section of diseased stem in clear water. A white milky stream of bacterial cells and slime flow from infected stems into the water after a few minutes.
Control of bacterial wilt in infested soils is difficult. Therefore, avoid using diseased transplants and establish plantings in non-infested soil. Soil fumigation may provide partial control, but does not completely eliminate bacteria from soil. When infected plants are identified, remove and destroy them immediately.

Figure 7. Bacterial wilt – sudden wilting without yellowing.
Pith necrosis
(Psuedomonas corrugata)
Pith necrosis has mostly been a problem in greenhouse tomatoes, but has been observed in field-grown plants in Oklahoma. It is currently of minor importance because levels of the disease have been low and the disease does not progress after initial infections are observed.
Symptoms of pith necrosis become evident when the first fruit cluster is approaching maturity. Individual branches or sections of plants turn yellow, wilt and die back. Rarely does the entire plant show symptoms. Elongated, firm, dark brown cankers are evident at the base of infected branches (Figure 8). Adventitious roots frequently develop on green areas of the stems surrounding the cankers. Splitting affected stems reveals a hollow chambered pith, which may have streaks of dark discoloration. The disease does not increase once the first symptoms are observed, and it may later become masked by new growth.
Little is known about sources of the bacteria, or how and when plants become infected. The disease is associated with low nighttime temperatures, high humidity and high nitrogen fertilization. No control strategies are available for pith necrosis.

Figure 8. Pith necrosis – stem canker with nearby adventitious roots forming.
Virus Diseases
Viruses are particles that are smaller than a single cell and not visible through a light microscope. Most viruses are spread by insects, but some are spread mechanically through exposure of plant wounds to infected sap. In insect transmission, plants become infected by the probing (sampling) and feeding activities of the insects such as aphids, thrips and leafhoppers that carry viruses (vectors). Once inside the plant, the virus multiplies and spreads throughout the plant. Virus infection causes a wide range of symptoms including unusual color patterns in leaves and fruit, distorted growth, plant stunting, reduced yield and plant death. Several different virus diseases of tomato occur in Oklahoma, some at damaging levels. Virus diseases are difficult to manage. Insecticide programs aimed at controlling the insect vectors have generally not been effective in reducing virus diseases because plants become infected quickly, before the insects succumb to the insecticide.
Alfalfa mosaic
(Alfalfa mosaic virus)
Alfalfa mosaic is a destructive disease of tomato because of the severe damage it causes to fruit. However, it is currently of minor importance because it occurs at low levels, mostly in tomatoes situated near old alfalfa fields. Symptoms of alfalfa mosaic are a bright yellow mosaic of newly expanded leaves (Figure 9) and an extensive browning and splitting of fruit (Figure 10). The virus has a wide host range, but infection in tomato is thought to arise from old alfalfa fields harboring the virus. At least 14 species of aphids transmit the virus from infected to healthy plants in sap that clings to their mouthparts. The virus can also be spread mechanically. Control strategies include not planting tomatoes adjacent to alfalfa, and removal of symptomatic plants to avoid mechanical transmission of the virus to nearby healthy plants.

Figure 9. Alfalfa mosaic virus – yellow mosaic in foliage.

Figure 10. Alfalfa mosaic virus – browning and splitting of fruit.
Curly top
(Beet curly top virus)
Curly top or western yellows disease is caused by one or more strains of the beet curly top virus. It is a destructive disease of tomato that has increased in importance in Oklahoma. Curly top was first reported in Oklahoma in 1945, but because the virus is uniquely difficult to detect using standard virus-testing methods, for some time the disease has been misidentified as psyllid yellows. Outbreaks of curly top have been cyclic. In years where curly top outbreaks are mild, only isolated plants are affected. However, levels of curly top have exceeded 50 percent in some years.
Symptoms of curly top begin as upper leaves become pale green and curled. All leaves eventually become curled and plants appear pale green and stunted as new growth is stopped (Figure 11). Leaves of affected plants become thickened and often have purple colored veins (Figure 12). Leaf stems and branches become brittle and are easily snapped. Plants affected early in the season are usually killed. On plants that develop symptoms after fruit set, fruit prematurely ripens and becomes dull red and wrinkled.
Beet curly top virus is spread long distances and transmitted to plants by the beet leafhopper. The virus is rapidly acquired by leafhoppers while they sample or feed on infected plants. The virus has a wide host range that includes more than 300 species of broadleaf plants. These include weedy species of thistle and mustard, which are suspected to serve as important reservoirs of the virus, and crop species including tomato, spinach, pepper and sugar beet. Once leafhoppers become infective, they remain so for life and can transmit the virus to healthy plants within seconds during their sampling and feeding activities. Tomato is not a preferred host for the beet leafhopper and it is thought that the virus is transmitted during brief visits while leafhoppers are searching for desirable hosts. Symptoms on tomato appear about two weeks after plants are first infected. Because the beet leafhopper does not feed on tomatoes, there is little or no secondary spread within tomato fields. The level of curly top in tomatoes is thought to correspond with leafhopper migration patterns and the proportion of leafhoppers within a population that are carrying the virus. Sources of the leafhoppers and the virus that affect tomatoes in Oklahoma are not currently known. In arid and semiarid areas of the western U.S. where curly top is a chronic problem, leafhoppers overwinter in dessert areas on winter vegetation. The beet leafhoppers acquire the virus from overwintering on hosts such as Russian thistle and wild mustard. During the spring when winter vegetation dries up, leafhoppers migrate into valleys in search of desirable host plants and infect various crop species during their search for food.
Control strategies for curly top are limited because insecticide sprays are not effective in reducing curly top and there are no resistant varieties of tomato available. Control efforts in tomato have centered on reducing the attractiveness of a tomato planting to the migrating leafhoppers. Leafhoppers are attracted to isolated plants in exposed areas of soil. Therefore, widely spaced transplants growing in open areas are highly attractive. Dense or double-row planting patterns, intercropping tall plants such as corn within tomato plantings, and situating tomatoes near structures and shade have been reported to reduce levels of curly top. Row covers that exclude insects early in the season may also be beneficial.

Figure 11. Beet curly top virus – leaf curl and stunting.

Figure 12. Beet curly top virus – purple leaf veins.
Tobacco mosaic
(Tobacco mosaic virus)
Several strains of tobacco mosaic virus (TMV) infect tomato and numerous other crops and weeds. The disease is of minor importance on tomato because of the widespread use of resistant varieties. Yield loss from TMV is a result of reduced number, size,and marketability of fruit. The virus may be introduced on contaminated seed, infected crop plants and weeds and tobacco products. TMV is very persistent and infective. It is spread primarily by humans handling infected plants and mechanically transmitting the virus to healthy plants via the sap. Symptoms of the disease are variable and depend on the virus strain present, the tomato variety grown and temperature. High temperatures tend to mask foliage symptoms. Typical leaf symptoms of common strains are mottled areas of light and dark green color (Figure 13). Infected leaves may also be small, curled and malformed. Susceptible varieties infected early are stunted and pale green in color. Fruit symptoms are usually absent, but may include various forms of uneven ripening. Severe strains may cause unusual mottling symptoms, rendering fruit unmarketable.
Virus-infected plants cannot be cured, so effective management relies on prevention. Resistant varieties, identified by the letters “TMV” in seed catalogs, on seed packages or on variety labels should be planted where available. Washing hands with soap and water before and during the handling of plants and after using tobacco products effectively inactivates the virus. Planting should be started with healthy transplants produced from virus-free seed. Seed treatments for elimination of the virus from seed include soaking seed for 10 minutes in a 10 percent solution of trisodium phosphate (Na3PO4) or dry heat treatment of seed for 2 days to 4 days at 150 F.

Figure 13. Tobacco mosaic virus – light and dark green mottling of leaves. (Photo courtesy M. Clerjeau, INRA France.)
Tomato spotted wilt
(Tomato spotted wilt and Impatiens necrotic spot viruses)
Tomato spotted wilt disease is caused by two closely related viruses, Tomato Spotted Wilt Virus (TSWV) and Impatiens Necrotic Spot Virus (INSV). These viruses infect many weed and crop plants, which then serve as new sources for the virus to spread. They are spread from infected to healthy plants by several species of thrips. Thrips are tiny insects that inhabit flowers, leaves and soil. Impatiens and gloxinia are commonly found to be infected with the viruses in greenhouses. In the field, several vegetable crops, winter annual weeds and perhaps infected thrips overwintering in soil are sources of the viruses. Tomato and pepper are among the most susceptible vegetable crops to tomato spotted wilt. In Oklahoma, tomato spotted wilt has been a problem mainly in greenhouse production of transplants. Planting of infected tomato transplants is thought to be responsible for the observed infections of field-grown tomatoes. In other southern states, field infection of tomato and other crops is common, and viruses have apparently become established in vegetation in and around fields. Yield losses in tomato can be extreme if numerous plants are infected early in their development.
Generally, symptoms of tomato spotted wilt on tomato are variable and include stunting of new growth, a brown spotting or bronzing of young leaves (Figure 14) and brown streaking on terminal stems. Dark purple to brown ringspots may develop in some leaves. Infections in older plants often do not affect all the terminals and may result in one-sided growth. Plants infected early fail to set any fruit, while those infected after fruit set produce fruit with ringspots and are off-flavor. Ringspots are light green in color on green fruit and a more conspicuous yellow on ripe fruit (Figure 15). Since disease symptoms are variable, suspect plants should be submitted to the OSU Plant Disease and Insect Diagnostic Laboratory for confirmation.
Control of tomato spotted wilt centers on exclusion of the viruses from transplant production systems and on establishing field plantings with healthy transplants. Avoid producing vegetable transplants in a greenhouse where ornamentals have been imported or are being vegetatively propagated. Enclosing greenhouse windows with fine-meshed screening (400 mesh to 1,000 mesh) can help reduce the movement of thrips into a greenhouse. Inspect incoming plants, especially ornamentals, for virus symptoms and thrips infestations. Remove and destroy any symptomatic plants. Controlling thrips with insecticides, particularly in the field, usually does not reduce disease, because thrips are able to infect plants before the chemicals can act to kill them. An intensive thrips management program in conjunction with other control tactics, may be more effective in a closed greenhouse system. Tomato varieties with resistance to tomato spotted wilt have been developed.

Figure 14. Tomato spotted wilt virus – spotting and wilting of upper leaves and shoots.
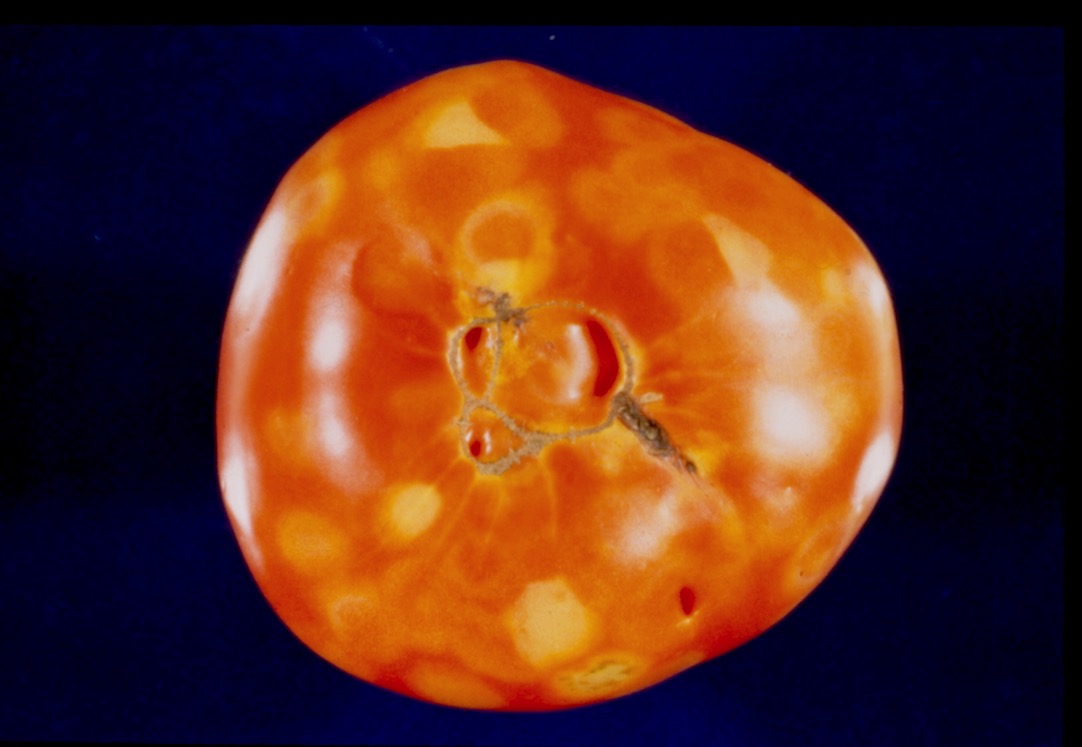
Figure 15. Tomato spotted wilt virus – ringspots on fruit. (courtesy F. Killebrew, Miss. State Univ.)
Nematode Diseases
Nematodes are microscopic round worms that live in soil. Some nematode species feed on plant roots and cause diseases. Repeated cropping of susceptible crops may result in nematode populations rising to levels that cause economic damage. Reduced plant growth and yield result from the poor root development and function caused by nematode feeding. Nematodes are most damaging in warm sandy soils. Poorly drained soils with high clay content often do not support high nematode populations. Low population levels generally do not cause damage, and may even stimulate plant growth.
Root-knot
(Meloidogyne spp.)
Root-knot is the most important nematode disease of tomato in Oklahoma. Four kinds of root-knot nematodes commonly affect tomatoes and many other crop plants. These are the southern, northern, peanut and Japanese root-knot nematodes. The northern and southern root-knot nematodes are most common in Oklahoma. The peanut root-knot nematode has been identified in Oklahoma, but it is confined to the far southwest corner of the state. Root-knot nematodes feed within roots (Figure 16) and susceptible plants, like tomato, respond to this feeding by developing galls or knots on affected roots. The northern root-knot nematode produces small, discrete galls while the southern, Japanese and peanut root-knot nematodes produce large galls and massive root swellings (Figure 17). Infected plants are stunted, appear yellow or pale green in color and wilt easily, even when soil moisture is adequate. Severe infestations can dramatically reduce yields and eventually kill plants. Damage from root-knot nematode feeding may also increase the incidence of other soilborne diseases such as Fusarium wilt and cause Fusarium wilt-resistant varieties to become susceptible.
Management of root-knot should focus on sanitation measures for preventing contamination of soils, reducing populations below damaging levels where infestations already exist and variety selection. Sanitation measures include planting nematode-free tomato transplants and avoiding the introduction of nematodes on any other type of transplant stock or with soil. This is difficult in reality, because soil clinging to plant roots may contain nematodes without obvious plant symptoms. Equipment and boots should be washed free of soil before working clean ground when moving from areas suspected of harboring nematodes.
Strategies for reducing nematode populations include starving nematodes by using a two-year crop rotation with grass crops that are resistant to root-knot nematodes, such as corn and milo. Soil solarization (see Extension Fact Sheet EPP-7640) may be effective in some situations, but soil fumigation provides more consistent control of nematode populations (see Extension Circular E-832). Soil fumigants are restricted-use pesticides that can only be applied by certified applicators. Incorporation of cruciferous green manures such as cabbage, mustard and rape into soil may also help reduce populations, particularly when combined with solarization. Before a susceptible crop is grown where nematode infestations are known to occur or are suspected, populations should be monitored by soil sampling and analysis to ensure that management practices have effectively reduced numbers below damaging levels (see Extension Fact Sheet EPP-7610).
Many root-knot resistant tomato varieties are available. These varieties have been bred for resistance to the southern root-knot nematode, but are probably also effective against the peanut and Japanese nematodes. These varieties are not effective against the northern root-knot nematode, which is prevalent in peanut-growing areas of the state.
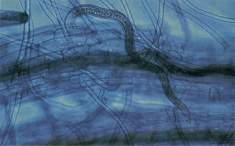
Figure 16. Nematodes feeding in a plant root.

Figure 17. Root-knot nematode – galls or knots on roots.
Reference
Jones, J.B., Jones, J.P., Stall, R.E., and Zitter, T.A. 1991. Compendium of tomato diseases. APS Press, St. Paul, 73pp.
Extension Plant Pathologist
Extension Vegetable Crops Specialist
