What is Clean in Place (CIP)?
Introduction
This fact sheet is a brief introduction to Clean-In-Place methods, commonly referred to as “CIP.” CIP is an automated method of cleaning food-processing equipment without disassembly using validated procedures. Equipment may be CIPed if a cleaning solution can be brought into contact with the surface(s) to be cleaned using spray heads or flooding. Flooded systems are normally agitated by hydraulic or mechanical means. Examples of equipment cleaned via CIP are tanks, piping systems, filter housings, ductwork, conveyors, homogenizers, centrifugal separators, and heat exchangers.
History of CIP
Manual cleaning was the state-of-the art for food processing equipment prior to the advent of CIP. Piping systems and food processing equipment were disassembled and cleaned by hand. After cleaning, parts were reassembled and sanitized. Equipment size was severely limited due to manual cleaning requirements. Pipe lengths of more than 10 feet were not possible because of handling limitations associated with manipulation of the pipe, power brush cleaning, and wash tank dimensions. Product storage tanks were limited to about 8 feet in height to allow an average-sized person to scrub ceilings and upper surfaces of walls with a brush on a pole.
The need for CIP systems surfaced as a result of World War II when shortages of metals forced dairies to substitute Pyrex glass tubes for metal pipes. Dairy owners and operators actively sought a method that would help them clean glass tubing without disassembly and associated breakage. The first automated CIP system was installed in a family operated dairy in 1953. Development was rapid and CIP became widespread in dairy plants by the mid-60s. The late Dale Seiberling was instrumental in the development of CIP and was involved with the first dairy system and non-dairy systems in the 1960s and the first pharmaceutical systems in the late 70s (Seiberling, 1997; Seiberling, 2008)
CIP Fundamentals
Functional Goals of CIP
- The functional goals of CIP include:
- Uniform, effective cleaning of equipment
- Prevention of product contamination with cleaning chemicals
- Improved personnel safety
- Increased productivity:
- Reduced downtime and process equipment maintenance
- Reduced expenses for energy, water, and cleaning chemicals
Processes Employed
Most CIP cleaning cycles include one or more of the following steps:
- Rinsing Washing
- Sanitizing
- Drying
Rinsing is often the first step of a CIP cycle. Rinsing uses water (heated or unheated) to remove loose soils and residues from surfaces. Rinse water may be recycled or sent to the waste drain depending on the concentration of soils and chemicals in solution and/or suspension.
Washing makes use of cleaning solutions (chemicals) and agitation to remove attached soils. The cleaning chemicals are carefully selected for effectiveness based on the soil type and the nature of the surface to be cleaned. Agitation may come from one or more of several sources including spray impingement, hydraulic flow, mechanical agitation (mixers), ultrasonics, and air bubbles.
Sanitizing is used specifically to kill and prevent growth of bacteria on surfaces of cleaned equipment. Pumpable or water-soluble agents are left in contact with surfaces to accomplish sanitizing goals. Drying is normally done using warm or hot air. The drying step removes water from product contact surfaces to prevent bacteria growth and dilution of product during startup.
A typical CIP cycle is a sequence of steps that are custom-designed to clean a particular food soil from a given set of processing equipment. An example of a CIP cycle is as follows:
- Pre-rinse
- Caustic wash
- Rinse
- Acid rinse
- Sanitize
- Post-rinse
Basic System Configurations
Two major types of CIP systems are in use today –single use and reuse. Single use systems (see figure 1, which shows a portable, single-use CIP system) discard all liquids to the drain after use. Reuse systems store cleaning fluids for reuse in subsequent cleaning cycles. Single use systems consist of the following major components:
- Accumulation tank
- CIP supply pump
- Heater
- Controls
- Sensors to monitor control variables
- Chemical feed equipment
Figure 1. Single-use CIP system. Photo courtesy of the Robert .M. Kerr Food and Agricultural Products Center, Oklahoma State University.
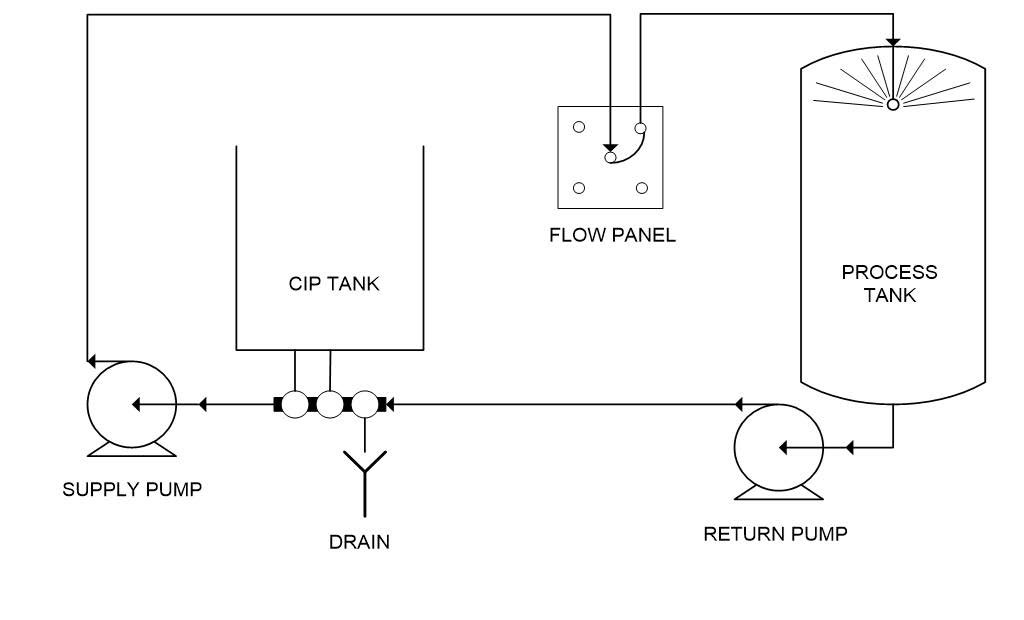
Reuse systems have all the same components as the single-use system with added holding tanks to store cleaning waters for reuse. A schematic of a typical reuse system is shown in Figure 2.
Figure 2. Simplified drawing of a CIP reuse system.
Operational Requirements
Control and documentation of five variables is critical to the operation of CIP systems:
- Temperature
- Maintenance
- Pressure
- Concentration
- Time
Temperature of the CIP solutions is determined by process requirements and cleaning chemical activity. Temperature is recorded throughout the temperature-critical steps of the process (e.g. wash).
Maintenance of turbulent flow in pipes is an important factor in cleaning effectiveness. Turbulent flow promotes hydraulic “scrubbing” of surfaces. As a general rule, a fluid velocity of at least 5 feet per second (1.5 meters per second) in pipelines will maintain turbulent conditions. CIP fluid flow rates are normally measured and recorded for process verification using a flow meter and a secure data recorder. Pipe geometry must also be carefully designed to reduce dead-ends, bubble formation, and ensure that the cleaning fluid completely wets surfaces for cleaning (Lloyd, 2008).
Adequate pressure in CIP fluid circulation systems is necessary for reliable performance of spray devices. Pressure also serves as an indication of flow (CIP solution pressure is proportional to the square of its velocity). Many CIP control systems record pressure for documentation of activity.
Generically, cleaning chemicals are either detergents (acid or alkali), sanitizers or disinfectants. Cleaning chemical concentration provides an important measurement of effectiveness. Chemical concentration can be measured manually or automatically using a conductivity probe. Optimal chemical concentration levels are normally recommended by the chemical supplier.
Cleaning cycle time refers to the amount of time that cleaning solutions are in contact with surfaces being cleaned while required conditions (temperature, flow, pressure, concentration) are met. Cycle times are experimentally determined and depend on many factors that include soil amounts and characteristics, surface geometry, chemical concentration and cost, line availability (for example, production and labor factors), composition of flexible seals and metal and plastic surfaces.
Spray Devices
Description
A spray device is a mechanism used to apply CIP fluids to a surface that requires cleaning. Two general types are available: static and dynamic. Static spray devices are motionless heads with drilled holes that represent nozzles. Popular versions include spray balls (figure 3), tubes, and bubbles. Spray balls, tubes and bubbles are essentially the named shape with drilled spray holes. Static spray balls are normally designed for 20 to 30 gpm (113 lpm) and 20 to 30 psi (138 to 207 kPa) pressure drop. The effective cleaning diameter of a static spray ball is about 8 ft (2.4 m).
Figure 3. Fixed spray balls, Photo courtesy of the Robert M. Kerr Food and Agricultural Products Center, Oklahoma State University.
Dynamic spray devices have a moving spray head, or body, which is driven by the cleaning media (figure 4) and/or mechanical means. Spray balls are frequently left in the tank during processing. Table 1 lists advantages and disadvantages of static and dynamic spray devices.
Figure 4. Fluid driven dynamic spray devices. Photo courtesy of the Robert M. Kerr Food and Agricultural Products Center, Oklahoma State University.
| Spray Ball Type | Advantages | Disadvantages |
|---|---|---|
| Static | Mechanically simple, low cost, low maintenance, simple to validate, directed flow paths (custom drilling) | Susceptible to clogging, lower impingement force |
| Dynamic | Superior cleaning action due to higher impingement forces and directed flow path(s | Mechanically more complex, higher cost, higher maintenance, more effort to validate, susceptible to clogging |
Table 1. Advantages and disadvantages of static and dynamic spray devices
Application
Fixed spray balls can be customized by drilling holes in a specific pattern and diameter(s) to match the cleaning requirements of the process equipment. Equipment that is carefully designed and installed can be cleaned with a minimum of strategically placed spray balls. Spray balls are placed to ensure that surfaces are wetted with sufficient cleaning fluid at adequate pressure.
Spray device coverage tests document proper fluid coverage of surfaces to be cleaned. Tests are performed by applying a coating of water-soluble dye (e.g. riboflavin) onto the surfaces to be cleaned, and then rinsing it off with water applied through the spray device(s). Riboflavin dye is easy to spot because it fluoresces under black light (see figure 5). Dye removal is visually observed and the system is adjusted until all dye is consistently removed. Spray device coverage tests are recommended while the equipment is still at the manufacturer’s shop, just after installation and after changes are made in the system.
Figure 5. Interior of a process tank that has been sprayed with a riboflavin solution that is fluorescing under illumination of black light. Photo courtesy of the Robert M. Kerr Food and Agricultural Products Center, Oklahoma State University.
Cleaning Agents
Generic Description
Chemical cleaning agents make use of mechanisms that include wetting, dissolving, saponification, emulsification, dispersion, and sequestering. Formulated cleaning compounds often include multiple cleaning agents. Wetting agents are found in most cleaning formulations; their purpose is to lower the surface tension of the liquid. Lower surface tension helps to increase the contact area between the liquid and the surface area being cleaned, reducing the amount of cleaning agents needed. Dissolution of residues is one of the primary mechanisms of cleaning agents. The objective is to dissolve the soil into the cleaning solution (e.g. sugar into water). Factors such as pH, temperature, agitation, and physical form of the residue affect solubility. Acids, bases, surfactants and solvents may be added to make soils more soluble.
Acidic additives (low pH) are used to dissolve soil residues. Phosphoric, acetic, citric, and nitric acids are examples of acidic cleaning agents. Saponifiers are alkaline substances with high pH values that hydrolyze fat to form soap and glycerol. Examples are sodium hydroxide, potassium hydroxide, soda ash, and tri-sodium phosphate. Emulsifiers break up liquid soils into smaller droplets that can be suspended in the cleaning fluid when the two do not mix (e.g. oil and water). Dispersants are similar to emulsifiers but act on solid particles. Sequestrents render minerals soluble. Chelantsare a type of sequestrant that are often used to remove minerals from unsoftened makeup water. Other cleaning agents include enzymes, catalysts, and solvents. Enzymes are serving an increasingly important role in the removal of biofilms on surfaces.
Matching Cleaning Agents with Process Conditions
Cleaning agents are often complex formulations of chemicals that are custom-designed for a specific situation. Typical soil residues include protein, carbohydrates, fat, salt, and organisms. Food contact surfaces in most processes consist of a variety of stainless steels, plastics and elasto-mers. A custom formulation starts from a base recipe that is adjusted then fortified with additives to address the end user’s needs. Examples of specific needs are unique soil residues, process equipment geometry and physical composition, water-quality, safety, environment, budget, and time available. Given the complexity and highly specialized nature of the task, formulation of cleaning solutions should be the responsibility of persons that have practical experience and in-depth knowledge of the subject.
System Validation
Acceptance Criteria
End users must determine their own acceptance criteria for equipment cleanliness. For single-purpose equipment without cross-contamination issues, a visual inspection may be adequate (Bismuth and Neumann, 2000). Multi-purpose equipment may require specific analytical acceptance criteria for target residues in the product and on equipment surfaces (LeBlanc, 2000).
Sampling Methods
Selection of sampling methods is critical to system validation. Samples must be representative of the system as a whole or in a worst-case scenario. Four types of sampling methods are direct surface, swab, rinse, and placebo. The state-of-the-art for direct surface sampling is visual observation. Swab sampling uses a fibrous material to wipe over the surface to remove residues. The residue is extracted from the swab and analyzed.
Rinse sampling involves taking a sample of the final CIP rinse water, or separate rinse water, and analyzing it for contamination. Placebo sampling originated in the pharmaceutical industry and involves manufacturing a placebo product (drug without the active substance) following a normal product manufacturing and CIP cycle. The placebo is analyzed for residual values of the active substance (LeBlanc, 2000). This technique is effective for validation of food processing systems when selected ingredients are removed from the product to make a “placebo”.
Analytical Methods
Valid analytical methods must be stable, accurate, precise and selective for the particular contaminants, and have a limit of detection of at least 25% of the target residue limit in the sample (LeBlanc, 2000). The chromatographic methods (LC/MS, GC/MS and HPLC) are often preferred for cleaning validation studies (Bismuth and Neumann, 2000).
Operations, Utilities and Maintenance
Many operations, utilities and maintenance issues are often overlooked in CIP systems with disastrous results. Gaskets are a chief example. Gaskets become worn and lose their elastic properties over time and should be replaced periodically. Over-tightening of gaskets (especially at leaky joints) can result in their extrusion into pipelines and product flow paths. Extruded gaskets form minute pockets, which are difficult to clean and may promote bacterial growth. Torque limiters, improved fittings (e.g. Swagelok’s TS Series Biopharm fittings), and better gasket materials help to solve these problems. Color-coded or labeled gaskets can aid gasket replacement procedures.
Steam used in CIP systems should be dry, clean and supplied at ample pressure. Condensate separators can be used to remove moisture before steam reaches control valves. Bypasses and shutoff valves should be included in systems to facilitate maintenance activities. Duplex strainers and dirt legs should also be included. Steam lines may need to be cleaned in systems where steam is injected directly into the product.
Compressed air is frequently used in CIP systems to operate controls, dry lines, vessels and equipment. Oil, bacteria, particles, and moisture should be eliminated from the system and adequate filtration and traps installed. UV lamps can be incorporated to kill bacteria. The compressed air system should be inspected and cleaned periodically.
Softened filtered water is recommended for CIP operations. Minerals in hard water can interact with soil, cleaning agents and stainless steel surfaces. Mineral content can vary through the year with water quality, causing process changes that are difficult to track and identify.
Stainless steel is not invincible. It can pit or corrode if subjected to iron (or other metal) contamination. Fluids high in chlorides or sulfur should not be in continuous contact with stainless steel. Cleaners or sanitizers should be rinsed from stainless steel surfaces after no more than 20 minutes of contact time. Avoid sudden temperature changes that could cause the stainless steel to fatigue and crack. Do not exceed the maximum recommended concentrations of chemicals. Initial passivation and frequent cleanup of stainless steel equipment is critical to ongoing performance.
The Future of CIP in the Food Industry
- Look for the following trends in CIP:
- More widespread use and mandated requirements for CIP processes
- More powerful and directed cleaning agents
- Faster, more focused CIP systems
- CIP systems that operate in parallel with production.
- CIP systems for general plant surfaces (e.g. walls and floors) and utilities (e.g. drains, compressed air lines).
- Food contact surfaces with finishes that resist attachment of food soils and bacteria
- Coatings for food contact surfaces that improve cleanability and discourage bacterial growth and attachment
- Improved sensors and equipment for control and validation
- Improved methods of mechanical cleaning
- Reduced water use
Sidebar
Ten concepts of CIP (Bowser, 2021):
- Time, temperature, flow, pressure and concentration are CIP operating variables that work together to clean and sanitize.
- Record CIP operating variables to verify cleaning and sanitizing.
- Design CIP systems in advance. Add-ons are expensive and rarely work properly.
- Conduct dye tests on vessels and equipment in advance and periodically.
- Eliminate piping dead-legs. Dead-legs are difficult to clean.
- Eliminate puddles in vessels. Puddles leave bathtub rings.
- Maintain turbulent velocities of cleaning fluids in pipes.
- Install equipment and piping to gravity drain. Wet spots may harbor bacteria.
- Do not over-tighten gaskets (even if leaking). Replace gaskets according to schedule. An extruded gasket may cause product recall.
- Trust your senses. Report and investigate system quirks immediately.
References
Bowser, T.J. 2021. CIP workshop materials. R.M. Kerr Food and Agricultural Products Center, Oklahoma State University, Stillwater.
Bismuth, G. and S. Neumann. 2000. Cleaning Validation, a Practical Approach. CRC Press, New York. Pp. 35-61.
LeBlanc, D.A. 2000. Validated Cleaning Technologies for Pharmaceutical Manufacturing. CRC Press, New York. Pp. 135-191.
Lloyd, D. (2008). Design and control of CIP systems. Cleaning-in-place: Dairy, food and beverage operations, 150.
Seiberling, D.A. 1997. CIP Sanitary Process Design. in Handbook of Food Engineering Practice. K.J. Valentas, E. Rotstein, R.P. Singh, editors. CRC Press. New York.
Seiberling, D.A. 2008. Introduction and Historical Development. In Clean-In-Place for Biopharmaceutical Processes. CRC Press, New York. Pp. 1-20.