Using Genomics Part II: Risk Management of Genetic Defects
In recent years an abundance of genetic defects in a variety of breeds have resulted in substantial associated economic losses. In this fact sheet, we will discuss how these defects are inherited, how to determine the probability of producing an affected calf, and strategies to decrease issues related to genetic defects in your herd.
Inheritance
The bovine genome is made up of 29 pairs of autosomal chromosomes plus the sex chromosomes. Genetic defects are a result of alleles that cause a lethal condition or that severely handicap the performance of an individual. Simply speaking, at any location in the genome where there is a mutation, there are two alleles, which can be thought of as alternate forms of a gene. One allele comes from the sire and the other from the dam. There are many mutations within the genome which cause no known effects. However, the ones which are of interest are either ones which cause small effects on phenotype (The animals’ performance or how it looks), typically for performance traits, and those which cause large detrimental effects on phenotype or are lethal. Most genetic defects in beef cattle are a result of recessive autosomal mutations. The term recessive autosomal reflects the mode of inheritance for these defects meaning the mutation is on one of the 29 autosomal chromosomes (not a sex chromosome), and it is not expressed as a phenotype unless the animal receives two copies of the damaged allele (one on each of the two chromosomes inherited from its parents). The inheritance of these genetic defects works exactly like the inheritance of horns or red coat color, which are also autosomal recessive conditions and which require two copies of the red (or horned) allele before a difference is seen in phenotype. Because these traits are recessive, possessing only one copy of each of these alleles (a defect, red color, or horns) is not enough to change phenotype, because it is masked by a dominant allele (normal condition, black color, or polled). These animals are often called carriers, because they carry a recessive condition, but do not express it.
In any mammalian genome (the size of most mammalian genomes is three billion base pairs), it is almost assured that there will be multiple recessive lethal genetic defects present within the DNA sequence. However, because they are recessive, there must be two copies in the genome (one from each parent) in order to see affected progeny. In practice, the pairing of two disease alleles rarely occurs when animals are not inbred, because each animal likely carries mutations that cause different diseases. One of the best strategies to avoid incidence of genetic defects is to avoid mating animals to their relatives, or to employ a planned crossbreeding system (but keeping carrier status in mind when mating animals that have common breed composition).
Determination of Risk
When the mode of inheritance is known, we can calculate the probability of producing an affected calf by knowing the carrier status of the parents. Because the normal allele is dominant, there is no way to visually determine which animals are carriers and which are not. The only way to make that determination is through genetic testing or through known parentage of an affected calf.
If the animal tests as normal for a particular genetic defect, it has two normal alleles (represented by NN) and if the animal is tested and determined a carrier, it has one normal allele and one disease allele (represented by Nn). If an animal has ever produced an affected progeny (has the defect shown by phenotype, represented by nn), they can automatically be determined a carrier (Nn) and do not need to undergo genetic testing. If an inherited disease/defect is lethal, all adult animals are either normal or carriers (NN or Nn). They cannot have two copies of the disease allele (nn) and live.
When the carrier status of the parents is known, we can calculate the probability
of producing normal, carrier, or affected progeny using what is called a Punnet square.
It is simply a square with four quadrants and works like a multiplication table. The
alleles for each possible parental gamete (sperm and egg) are placed around the outside
and the letters are matched on the interior squares and the percentages are multiplied
together to determine the probabilities of each status in the progeny. To illustrate
this point, consider the following examples:
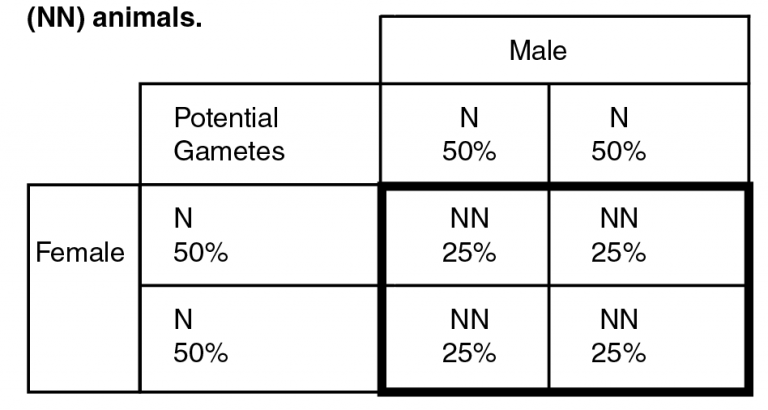
Example 1. Punnet square for the mating of two normal (NN) animals.
In the example shown on page 1, the two parents tested as normal, and do not carry any disease alleles. Because each gamete will contain one of the two parental gametes, each one will be seen approximately 50 percent of the time. Both parents are normal, so we have N gametes 100 percent of the time and all progeny (inside the bolded square) will have all normal alleles. This result illustrates why animals can be declared “clean” through pedigree. If the sire and dam have both been tested and determined carrier-free, any progeny of the mating will be free of disease alleles and do not need to undergo genetic testing.
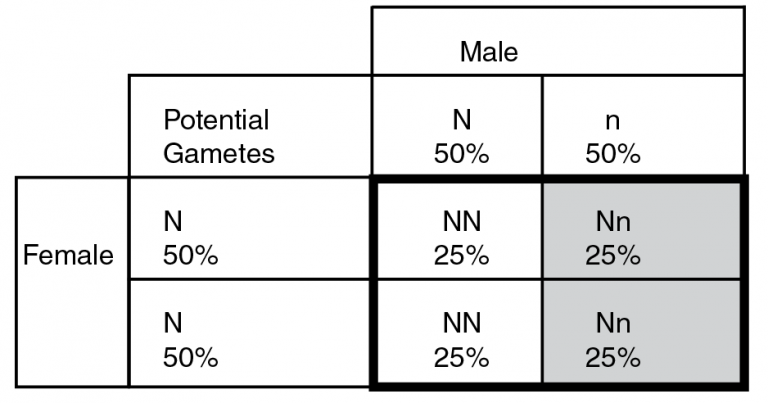
Example 2. Punnet square for the mating of one normal (NN) and one carrier animal (Nn).
In example 2, we have a mating of a carrier bull to a normal female. The female’s gametes will assort the same as in example 1, and she will always contribute N (normal) alleles to her progeny. The sire, however, can contribute either a normal (N) or disease (n) allele to his progeny. Each allele will occur in approximately 50 percent of his gametes, meaning ½ will be normal and ½ will contain the disease allele. When we match the possible gamete combinations (seen inside the bolded square), we can see that ½ of the calves (50 percent) will have two normal alleles (left side of the bolded box) while the other 50 percent of calves will be carriers (Nn; on the right, in light gray). In this scenario, we never see any affected calves, but they do have the potential to transmit the disease alleles to progeny in the next generation.
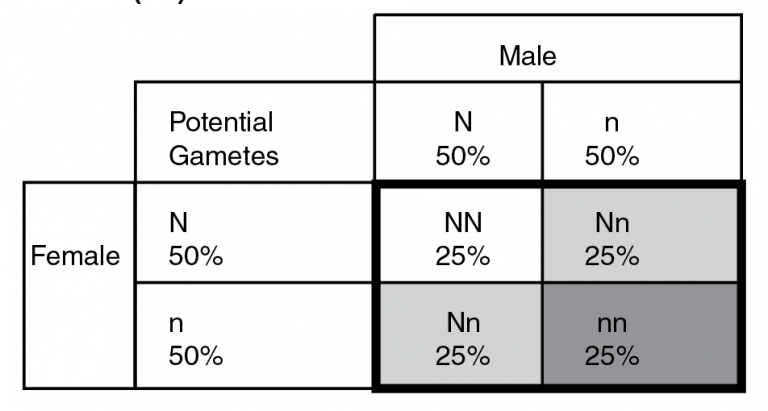
Example 3. Punnet square for the mating of two carrier animals (Nn).
The only time affected calves are generated is when two carrier animals are mated together, such as illustrated in example 3. Each sire and dam produces 50 percent gametes with normal alleles and 50 percent with disease alleles. When combined together in the progeny (bolded black box) all three genotypic classes can be present. This can be interpreted in terms of each progeny or in terms of a group of progeny produced from a group of carrier sires and dams. In the former, each progeny from the mating would have a 25 percent chance of having two normal alleles, a 50 percent chance of being a carrier (light gray), and a 25 percent chance of being affected (dark gray). In a group scenario (i.e. a large number of full-sib flush mates), we would expect 25 percent of progeny to have two normal alleles, 50 percent to be carriers and 25 percent to be affected. Knowing the carrier status of the herd through genetic testing makes effective risk management possible.
Strategies to Manage Risk of Genetic Defects
Whether or not there is a genetic test for the defect you are interested in managing will dictate how the incidence of genetic defects can be managed. If there is a test available for the genetic defect, it is advantageous to test those animals which have never had an affected calf, placing special emphasis on testing individuals which have carrier animals in their pedigrees. There is no need to test any animal that has parented an affected progeny since they are known carriers or is out of two animals that are tested “clean.” If an animal is genetically superior in many other ways, but is a carrier of a genetic defect, they do not need to be culled immediately. As you saw from the Punnet square examples above, as long as a carrier is always mated to an animal that has two normal alleles (homozygous normal), they will never have affected progeny. However, those progeny will then need to be tested to determine if they are carriers. It is a good risk management practice to test any animals that will be retained, such as replacement females. As a general rule, animals scheduled for culling do not need to be tested.
If you have seedstock, be cognizant of any breed registry requirements relevant to
an animal’s carrier status. Some breed associations do not allow carrier animals to
be registered, and most will require testing to determine carrier status if there
are carriers in the animal’s pedigree, provided that a genetic test exists. Often
progeny of animals that are carriers can be registered if the genetic test shows the
animal itself is not a carrier. It is much more effective to know these requirements
before making decisions regarding replacement females or culling, rather than suffering
the consequences after the fact. Breed associations do a very good job of identifying
and clearly displaying the status of carrier animals on the breed association webpages
(Figure 1).
Figure 1. Example of a carrier animal and its pedigree clearly identified by the American Angus Association. Carrier status on an animal that has undergone genetic testing can be found on the corresponding breed association web page for any animal. (Figure courtesy of Brian Freking)
Commercial cattlemen may find it easier to manage the genetic defect, rather than indiscriminately cull animals. For example, if a known normal (NN) bull is purchased, the carrier status of females may not matter because you will have 100 percent normal calves with an autosomal recessive defect. Alternatively, if you are using a bull in one breed on cows of another breed in a crossbreeding program, the likelihood of encountering the same defect in two different breeds is extremely small. Because of this, crossbreeding can be an effective risk management strategy for genetic defects. However, it should be noted that care must be taken when keeping replacement females and utilizing bulls of the same breed in subsequent years so that no affected calves are produced.
Testing Procedure and Availability
If you would like to order DNA tests for genetic defects, there are two main providers
for beef cattle. You can obtain more information on testing procedures, pricing, and
a list of available tests on their websites. If you are a seedstock producer, you
may need to submit the sample to the breed association rather than directly to a testing
company, so please contact your breed association to find out more information on
their guidelines for genetic testing.
Igenity
Testing Services:
http://www.igenity.com/beef/profile/Abnormalities.aspx
Contact Information:
http://www.igenity.com/ContactUs.aspx
Zoetis Animal Genetics
Testing Services:
https://online.zoetis.com/US/EN/Pages/Animal%20Genetics.aspx
Contact Information:
https://online.zoetis.com/US/EN/Products/Pages/AllAboutPfizerAnimalGenetics.aspx
Knowing how genetic defects are inherited can assist in making good management decisions both to preserve genetic progress and reduce or eliminate economic losses associated with genetic defects. In addition, awareness of genetic defect testing and how to calculate the risk of producing affected progeny will help to analyze risk and manage the incidence of genetic defects and their associated economic losses in the herd.
Megan Rolf
Former Assistant Professor