Honeybee Diseases and Their Recognition
Introduction
Honeybees provide essential agricultural services that include pollination of more than $15 billion worth of crops in the United States annually (USDA 2018). Without honeybee pollination activity, many important food crops could not provide the yields necessary for sustaining human and animal populations worldwide (Aizen et al. 2009). In addition to their economic impact, working with honeybees is an enjoyable activity for many beekeepers. However, beekeepers must closely monitor the surrounding environment for food supply factors such as nectar and pollen availability, which directly impact bee health and colony success.
Beekeepers must also remain alert for diseases that affect bees and can quickly spread and decimate hives. It does not take long for a disease to spread and cause the hive to collapse. Therefore, a biweekly hive health check is beneficial during spring and fall, especially during peak honey production. Early detection of diseases and other potential problems within the hive enables beekeepers to address problems before they cause extensive damage and mortality, leading to costly setbacks. This fact sheet provides information on the main diseases beekeepers could encounter in a honeybee colony. A future fact sheet will include pests that beekeepers need to recognize.
Honeybee Biology
Types of Honeybees
- Queen: Reproductive female of the hive. Usually there is only one queen. Almost all other bees within the hive are her offspring.
- Worker: Non-reproductive female bee. Worker bees have many functions throughout their life and comprise the bulk of the colony population.
- Drone: Male bee. His purpose is to breed with virgin queens. Drones serve little other
function within the hive. They are not as necessary as worker bees for hive activities.
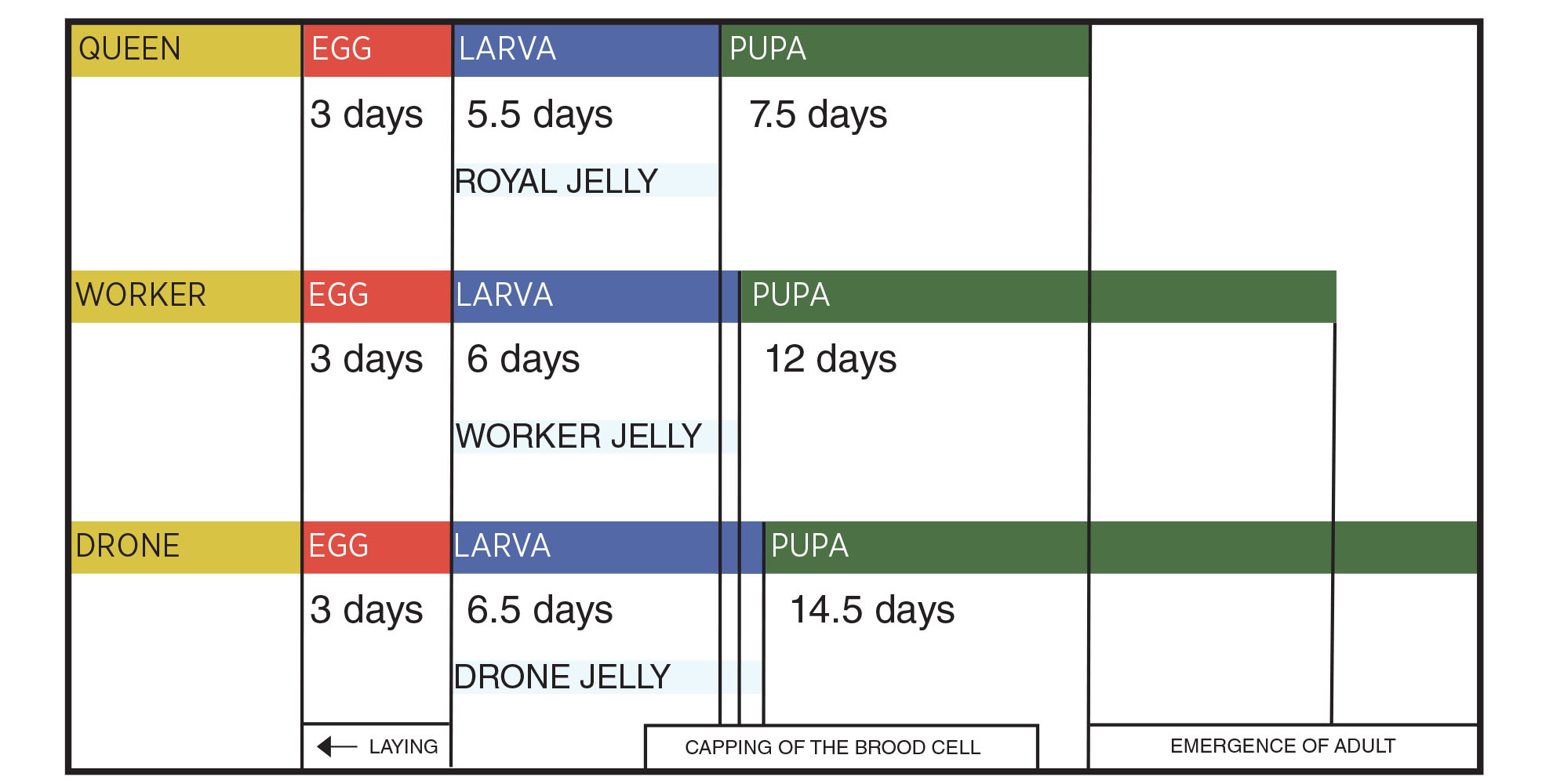
The number of drones varies based on the time of year. - Brood: Developing bees in their egg, larval or pupal stage. Knowing the stages of bee development will help the beekeeper better understand the progression of diseases discussed in this fact sheet. All bees go through four lifecycle stages: egg, larva, pupa and adult. The length of time within each stage differs depending on the type of bee: queen, worker or drone. Honeybee life stages are shown in Figure 1.
Honeybee Stages and Duration of Development
Figure 1. Honeybee development and life stage duration for each type (Vidal-Naquet 2015).
I. Viruses
Deformed Wing Virus
[Two versions; Infection rate 90-100%]*
- Deformed Wing Virus (DWV).
- Varroa destructor Virus-1 (VDV-1).
*[Infection rate of 90-100% is based on the percentage of hives surveyed that contained some diseased honeybees]
Figure 2. The arrow points to deformed wings of a worker. Note crumpled appearance of wings compared with wings of normal workers (Photo Credit: David Thompson).
*90-100% of hives surveyed contained honeybees infected with one or both viruses (USDA
2018).
Symptoms
Symptoms appear during late summer and fall. Wings are often deformed and stubby,
and affected bees cannot fly (Fievet et al. 2006; Fig. 2). Usually, the abdomen
is discolored and shortened. Guard bees may remove deformed bees from the hive. Pupae
often die and may remain in uncapped cells. If uncapped, cells may show
signs of having been chewed on by other bees (Ribiere et al. 2008).
Biology
Adult honeybees are virus reservoirs. This virus is transmitted directly to honeybees by the Varroa mite (Varroa destructor). As the Varroa mite numbers increase the spread of the virus also increases. Therefore, regular sampling for V. destructor is important. The virus reproduces and proliferates within the mite (Yue and Genersch 2005). Research suggests that this virus can also be spread directly by an infected queen to her eggs and may also be sexually transmitted by drones. Varroa destructor is the primary vector for this virus to spread into new honeybee hosts and colonies (Yue et al. 2007).
Treatment
Control measures for Varroa mites are essential for maintaining a healthy hive. To retard or stop virus spread, mites can be killed using commercially available insecticides called acaricides. Treatments should be applied before the honeybee colony enters its overwintering stage (Desai et al. 2012). Some commercial acaricides are formulated to slowly emit toxic miticidal vapors from acaricide-containing plastic or other types of absorbent strips. These strips emit mite-killing vapors while not harming honeybees. Alternative control measures include chemicals and essential oils that do not harm honeybees and can be sprayed inside the hive to help control mites. Chemical control methods will be the subject of future fact sheets. It is important to read label instructions carefully before the use of any mite treatment product. Important label instructions include temperature restrictions for application, restrictions on removal of honey for human consumption after treatment, the number of treatments and the time interval between sequential treatments.
Acute Bee Paralysis Virus (ABPV)
[Infection rate: 30 to 40%]
Figure 3. Hairless bees in the center of the group are suffering from acute paralysis. (Photo
Credit: Giles Budge). Arrows indicate workers with hairless bodies.
*30-40% of hives surveyed contained honeybees infected with ABPV (USDA 2018).
Symptoms
Honeybees are seen walking on the ground (usually in large numbers) around the hive rather than flying and gathering at the hive entrance. Abnormal positioning of wings is apparent where the wings are at different angles. Wings may exhibit trembling. Loss of abdominal hair also occurs along with a greasy look to the bee’s abdomen (Fig. 3). Abnormally high mortality of brood in capped cells also occurs (Bekesi et al. 1999). ABPV also causes early death of infected honeybees.
Biology
ABPV reproduces and accumulates in the brain as well as food-producing glands of honeybees. The virus usually kills infected larvae. Adults and larvae that become infected by transmission of the virus from Varroa mites die quickly. Acute paralysis alone rarely kills entire colonies but when transmitted by Varroa mites ABPV can cause colony collapse (De Miranda et al. 2012).
Treatment
Spread of the virus can be reduced or prevented through good sanitary practices, good husbandry and controlling Varroa mites using methods discussed above (Vidal-Naquet 2015). Sanitary practices include cleaning gloves and hive tools with alcohol before each new hive is inspected for disease and freezing stored honeycomb for at least 24 hours to kill disease organisms. Use only sanitized (flame treated or chemically disinfected) hive components and minimize transfer of frames between active hives.
Israeli Acute Paralysis Virus (IAPV)
[Infection rate: 10%]
Symptoms
IAPV can cause loss of the honeybee colony. In experiments within a controlled environment, IAPV caused bees to darken in color and become hairless and caused trembling of wings like ABPV (Maori et al. 2007).
Biology
IAPV attacks all life stages of honeybees, causing widespread infection throughout
the colony. The virus is transmitted by Varroa mites. IAPV is present throughout all
parts of the honeybee body but is concentrated in muscle, fat, and brain tissue. Infected
bees often show few signs of the disease although large numbers are infected. Eventually,
many bees show symptoms, leading to high mortality
within the colony.
Treatment
The Varroa mite is the main vector for IAPV and should be controlled with acaricides, vapor strips and hive sanitation and maintenance methods as used for the other earlier mentioned viruses (Maori et al. 2007).
Sacbrood Virus (SBV)
Figure 4. Tip of SBV-infected larva within its cell (Photo Credit:
Rob Snyder)
Figure 5. SBV-Infected larva (in its sac) removed from its cell.
(Photo Credit: Mitchael E. Wilson).
Symptoms
SBV occurs because of food deficiency, typically during spring and early summer when brood rearing is at its highest and nurse bees are spread thin. The virus is directly transmitted by bees tending to larvae, contaminated bees returning from foraging, and beekeeping activities. The virus can spread rapidly, killing thousands of workers. Symptoms can be difficult to identify because healthy bees will quickly remove dead bees and dead brood from the hive. If present, the virus will cause fluid to build up in the sac around a dead larva, producing a pale-yellow appearance (Figs. 4, 5). The sacs are full of SBV liquid and are fragile and easily broken open, spreading the virus (Ball 1999). If primary infected dead bees are not removed from their cells, they soon become dark and sticky. Eventually, nearby healthy larvae become secondarily infected by contact with primary infected larvae, and then die, dry out and shrink to form a flattened, curved, gondola-shaped scale within their cell (De Miranda et al. 2012).
Biology
SBV is a common disease of apiaries worldwide. Generally, the virus is not fatal to a colony. However, SBV weakens colonies and slows their growth. SBV becomes particularly concerning when associated with severe Varroa mite infestations. The fluid-filled sac that forms around the bottom portion of the larva is densely contaminated with the virus, although the amount of virus decreases significantly after a few days (Fig. 5). Within hives, asymptomatic adults occur first, spreading the virus throughout the hive as they prepare food for brood. In adults, SBV shortens lifespans, causing workers to become foragers earlier than usual, disrupting normal colony operations. SBV-infected foragers prefer to collect nectar over pollen and may stop harvesting pollen that is an important protein resource for developing larvae. SBV disease also decreases royal jelly production within a colony (Ball 1999; Blanchard et al. 2014). Honeybees use royal jelly to feed bee larvae and to create new queens.
Treatment
Sanitary practices and providing food supplements, particularly protein, to prevent starvation is usually enough to help a colony recover. Careful removal of infected brood and control of Varroa mites is also helpful in controlling SBV. If infection of brood increases to 20% or more of the brood population, the entire colony should be burned to prevent spread of SBV to other colonies (Vidal-Naquet 2015). Sanitizing all equipment and tools and cleaning hives will limit SBV spread. Clean, sanitized hive boxes and frames should always be used when establishing new hives.
II. Bacteria
American Foulbrood (AFB)
Figure 6. Sticky, stringy liquified larva in a cell, domonstrated by the 'matchstick' test (Photo Credit: Karpenko Natalia).
Figure 7. Depressed, concave brood cell caps, some punctured
(arrows) as typical of AFB (Photo Credit: Rob Snyder).
Symptoms
AFB is a devastating disease that can infect many native pollinators and honeybees alike. In its early stages, only a few honeybee brood cells may be affected. Therefore, AFB can be difficult to identify early in the disease process. When infected brood cells are opened, they contain a creamy-todark brown liquid. By using a toothpick or matchstick to probe a brood cell during an inspection, a dead larva can be drawn out of its cell along with the dark brown sticky, stringy liquid threads. This is referred to as the matchstick test (de Graaf et al. 2006; Fig. 6). Caps of infected brood cells become concave and punctured (arrows; Fig. 7). As severity of the infection increases the colony population will substantially decrease. Some colony members may become either lethargic or aggressive. The hive will have a distinct bad-smelling odor, giving the disease the name foul (Hansen and Brodsgaard 1999).
Biology
AFB is caused by a gram-positive, spore-forming bacterium. It is found in wax, honey, pollen and on the hairs of infected honeybees. AFB spores can live for 3-10 years on most surfaces within the beehive but can survive up to 35 years. The ability of AFB bacteria to survive in many areas of the hive facilitates disease spread because robbing or scavenging honeybees from other hives become contaminated and transport bacteria upon returning to their colonies (Genersch 2010).
Treatment
The Oklahoma Apiary Act and Rules (OAA 2005) regulates bee husbandry and disease control
treatments in Oklahoma. Upon discovery, all AFB infections must be reported to the
Oklahoma Department of Agriculture, Food and Forestry (ODAFF). Test kits for disease
identification can be purchased online (Extension Bee Health ).
Official test kits are strongly recommended to test for AFB and other bee diseases.
Multiple tests are recommended as AFB has been found coexisting with a related bacterial
disease, European foulbrood. If the hive is not yet thoroughly weakened and AFB is
caught early, ODAFFapproved antibiotic or chemotherapy treatments may be used. If
the colony is too weak to recover, the frames, comb, associated materials and bees
must be burned in an open pit. Disinfecting all salvageable parts of the hive may
be done using a blow torch to scorch their wood and other surfaces. Equipment, bee
suits and hands should also be disinfected.
Due to implementation of the Veterinary Feed Directive (VFD) the use of certain antibiotics is required to be done under the supervision of a veterinarian (FDA 2023). Currently, American foulbrood and European foulbrood are the only honeybee diseases that require veterinary consultation. Multiple methods of chemotherapy are also available. AFB exhibits widespread resistance to the antibiotic Oxytetracyclin (OTC) and is also resistant to hydrochlorides and Sulfathiazole. AFB is not strongly resistant to the antibiotics Terramycin or Tylosine. If an AFB-infected hive is treated and the AFB bacteria show resistance to Terramycin (Oxytetracycline), Tylosine treatments may be used as an alternative. To prevent build-up of bacteria resistance to a chemical treatment and to ensure effective treatment of bacterial infection, it is important to follow label directions during applications. Oxytetracycline extender patties have also proved an effective treatment where the OTC extender grease patties are placed inside the hive to introduce the chemical (Alippi 1999).
European Foulbrood (EFB)
Figure 8. Various stages of EFB in pupal cells. (Photo Credit: Georgia
Department of Agriculture)
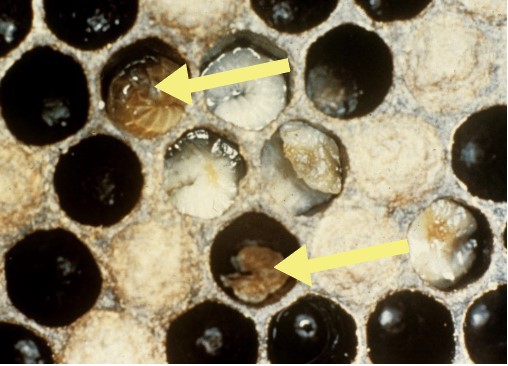
Symptoms
EFB targets and kills non-capped brood, usually within 4-5 days, leaving them stretched and displaced in their cells. Infected larva usually become soft and initially turn from white to yellow and eventually to a brown color (arrows; Fig. 8). Larvae are always killed before pupation, generally 1-2 days before capping. The larvae dry out and shrink, forming malleable flat scales within their cells. The brood pattern becomes spotty and erratic, and in severe cases symptoms can resemble AFB. When large portions of brood become infected the colony emits a sour odor. EFB most commonly occurs during late spring to mid-summer (FERA 2013).
Biology
EFB spreads in a hive through contaminated brood food. It is caused by a non-spore-forming bacterium and is carried and transmitted by infected adult bees. Therefore, bee trading amongst beekeepers, as well as interactions with naturally occurring swarms are among EFB’s main ways of spreading. Honeybee worker-drift between colonies also spreads the disease. Worker-drift occurs when honeybee workers forage and then return to the wrong hive, most often neighboring hives within the same apiary. Generally, EFB only becomes noticeable in colonies experiencing protein deficiency. It is thought that EFB bacteria that are infecting the larvae starve them of protein (Forsgren 2010).
Treatment
In moderate cases EFB can be treated by feeding protein resources such as pollen patties. Colonies with larvae provided extra protein sources usually survive EFB infection. If EFB persists after nectar flow, it may be necessary to re-queen the colony. Nectar flow refers to the period during the growing season when plants are in bloom and nectar is abundant for feeding by bees. In severe cases, the hive should be sanitized and destroyed as described above to prevent further EFB spread (Forsgren 2010). Oxytetracycline (Terramycin) is the only FDA-approved drug for the treatment of EFB (AVMA 2017). Off-label use of antibiotics is not allowed under the VFD. Therefore, although antibiotics other than mentioned already in this fact sheet have been used in the past, use of some of these is no longer permitted (AVMA 2017). Since EFB is less severe than some other bacterial diseases of bees, many beekeepers will try alternative approaches prior to resorting to the application of antibiotics.
III. Fungi
Nosema apis
[Infection rate 25-30%]
Figure 9. Fecal excrement at the hive entrance commonly seen in dysentery that accompanies Nosema apis infections (Photo Credit: Georgia Department of Agriculture).
*25-30% of hives surveyed contained honeybees infected with Nosema
apis (USDA 2018).
Symptoms
Nosema apis infections cause weak honeybees and high mortality in early spring populations
within hives. Secondary infections can occur from an accompanying
pathogen, the black queen cell virus. Lowered honey production by the worker population,
and dysentery in the honeybee population is apparent. This is noted by pale or brown
excretions on the bottom of comb or at the hive entrance (Fig. 9). Workers may also
be unable to fly and found to be crawling. Some workers have swollen, bloated abdomens.
The queen may also become infertile (Kilani 1999).
Biology
Nosema infections in adult honeybees reach their peak during fall and infections continue to become increasingly more severe during winter-to-early spring when bees confine themselves to the hive and face food resource scarcity. Nosema can also take root during other periods of prolonged confinement and food scarcity such as during multi-day rainfall events. Infection occurs through spores in feed, water, fecal matter, and through bees exchanging fluids orally (trophallaxis; Kilani 1999). Spores can remain viable for up to 1 year on a comb. When ingested by honeybees, Nosema damages walls of the midgut, leading to the death of the infected bee, often outside of the hive. Infections occur through spores in feed, water, and fecal matter and during feeding among bees and can lead to colony collapse (Chen et al. 2009).
Treatment
Use of sanitary practices and limiting comb trade between hives help prevent disease spread. Supplemental protein feeding helps strengthen bees before winter months. Although some beekeepers have treated Nosema with fumagillin in the past, this drug is not approved for use in the United States (AVMA 2017). Because bees are considered a food animal, use of non-approved drugs is strictly prohibited (AVMA 2017). Treatments with Thymol (antimicrobial properties), a component of Thyme oil, and Resveratrol, a component from grapefruit, grapes and blueberries, are also useful when combating Nosema infections (Vidal-Naquet 2015).
Chalkbrood
Figure 10. Chalkbrood in cells (Photo Credit: University of Georgia)
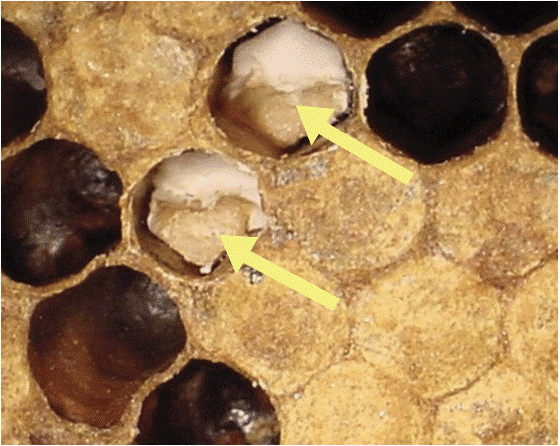
Symptoms
Larvae die in their capped cells, producing a pale-yellow color, with the cells becoming soft to the touch (Fig. 10). Cells eventually become covered over with white fungal mycelium and become dry, while larvae become white (arrows) or black dry mummies. Brood cells are often punctured and covered with white or black mold. Larva covered in mold may be removed to the outside of the hive by worker bees (Puerta et al. 1999).
Biology
Chalkbrood is a common disease in beehives. It is often asymptomatic in pollen stores. It is rarely fatal to a hive and usually only occurs when other stressors are affecting the hive. It affects primarily 1- to 4-day-old larvae. Chalkbrood occurs more frequently in cold, damp, shadowed apiaries with poor ventilation (Puerta et al. 1999).
Treatment
Currently, there are no known effective treatments to eliminate chalkbrood disease. If the disease occurs in your hives, introduce extra protein food sources and re queen with a more hygienic or disease-resistant strain of honeybee. It is also possible to transfer the bees to a pathogen-free hive or replace contaminated hive frames with clean frames. Hives with many infected cells should be removed from service and all hive materials sterilized (Aronstein and Murray 2010).
IV. Pesticide Contamination
Symptoms
Sometimes honeybees are exposed to pesticides while foraging. If pesticide contamination of bees occurs there will be a decrease in the number of workers and far fewer eggs laid. Additionally, pesticides can be absorbed into the honeycomb and food resources within the hive. If honeybees are dying without any apparent disease symptoms, pesticide contamination may have occurred. The first step to take where possible is to reduce pesticide applications near hives and flowers bees forage upon. Another step is to remove the old honeycomb that may have become contaminated with pesticides. Communicate with growers and farmers that are utilizing your hives about potential risks that insecticides and other pesticides pose to bees. To avoid pesticide drift and contamination of non-target areas, pesticide applications in field areas must not be done on windy days and must not be applied in areas with hives or on plants where bees are foraging. Pesticide exposure impairs honeybee physiological and behavioral systems, including navigation, memory, behavior, immune system function, thermoregulation and muscle efficiency, which can cause mortality and colony decline and collapse (Belzunces et al. 2012; Desneux et al. 2007; Henry et al. 2012).
Acknowledgement
We thank W. Wyatt Hoback, Dwayne Hunter and Marten W. Brienan for critical reviews that significantly improved this Fact Sheet.