Ethanol Fuel Basics
What is Ethanol?
Ethanol, or ethyl alcohol (CH3CH2OH), is a clear liquid that is typically made by fermenting vegetable matter. Ethanol is the alcohol found in beverages such as beer, wine, and distilled spirits. Civilizations have been making this type of alcohol for quite some time. In fact, people in the Indus Valley (parts of modern India) were fermenting grains and fruits to make alcohol beverages as long as 12,000 years ago1.
Ethanol is also a combustible compound and was used to power lamps in the mid 1800s. Ethanol also makes an excellent fuel for internal combustion engines. Henry Ford’s 1896 Quadracycle was originally designed to run on ethanol. Ford’s first successful commercial vehicle, the Ford Model T was specifically designed to run on gasoline and ethanol mixtures. In 1906, Henry Ford predicted that ethanol was the fuel of the future. Inexpensive imports of foreign oil and various domestic taxation laws in the early twentieth century may have forced ethanol into the background as petroleum products became the primary motor vehicle fuels.
1 Alcohol and Pleasure: A Health Perspective By Stanton Peele, Marcus Grant. Page number 102. Contributor Stanton Peele, Ph. D., J.D. Published 1999. Psychology Press. Self/Help. 419 pages. ISBN 1583910158.
Why an Interest in Ethanol Fuels – Again?
The United States imports approximately 60 percent of the petroleum used to make fuels and other petro-products. Of that percentage, approximately 44 percent of U.S. imported oil is from the Organization for Oil Exporting Countries (OPEC). Several of the OPEC countries have expressed hostilities toward the United States including threats of limiting oil exports. These are not new threats, and cut-offs occurred in 1973 when some countries of OPEC (OAPEC, or Organization of Arab Petroleum Exporting Countries) placed an embargo on petroleum sold to the United States, Great Britain, and Japan for their support of Israel during the Yom Kippur War. The price of fuel and petroleum-based products soared during this period. In response, President Nixon initiated the “Project Independence” program in 1974 to make the U.S. independent of imported oil. Much of the 1978 Energy Act was a concentration on the viability of domestically produced ethanol to offset imported oil.
A couple of years ago, the price of petroleum dropped dramatically (almost 70 percent, to less than $50 per barrel). However, petroleum is expected to resume its steady price climb at some point in the near future as worldwide use and decreasing production dynamics return to the market. Even with the recent drop in oil prices, the International Energy Agency predicted (November 6, 2008) that the price of crude would reach $200 in nominal dollars by 2030 due to supply and demand pressures3.
Ethanol is still seen as a way of reducing dependence on imported oil used for vehicle fuels. Some estimates have domestic use of fuel ethanol replacing more than 30 percent of gasoline demand by 20304. While complete independence of the U.S. from foreign oil may not be possible, ethanol could play a significant role in lessening the demand for imported fuels.
3 IEA doubles forecast for oil price by 2030, Associated Press, November 6, 2008.
4 http://www.afdc.energy.gov/afdc/ethanol/basics.html.
How is ethanol produced?
There are three primary methods of producing ethanol:
- Starch Fermentation
- Sugar Fermentation
- Cellulosic Fermentation
All three production methods rely on micro-organisms (yeast or anaerobic bacteria) at some point to produce ethanol as a biological by-product. How the processes reach the fermentation stages and the resulting production efficiencies are quite different. Let’s examine all three.
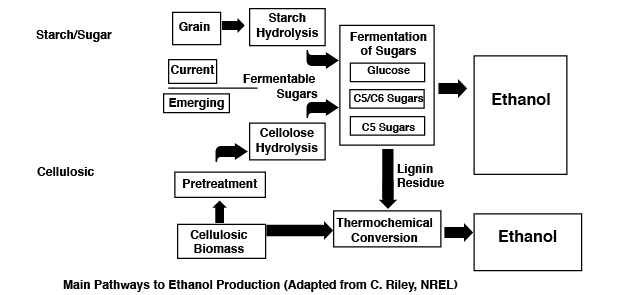
Starch Fermentation
In this chemical process, corn or other starchy grains, are crushed and mixed with water. Enzymes added begin to break down the long starch molecules into smaller sugar molecules. During a second addition, yeast is added to the sugar mixture. The yeast uses the sugars as a food source and produce ethanol and carbon dioxide (CO2) as waste products. A distillation process separates the more volatile alcohols from the watery mix. With repeated distillation and a filtration step, the end result is 100 percent (200 proof) ethanol.
The advantage of starch/sugar fermentation is that it is a relatively simple and well understood process (there are even simpler ethanol processes however). The use of corn as a fuel stock can benefit domestic agriculture. A disadvantage is the use of large quantities of food grains or farm land area to produce vehicle fuels, potentially driving up the price of certain foods. Another potential disadvantage is that the energy output contained in the ethanol may not be significantly higher than the required energy input (e.g., equipment and fertilizer energy).
Sugar Fermentation
In this version of ethanol production high sugar plant sources such as sugar cane or sweet sorghum are crushed and pressed to release sugar water. This sugar water is then directly fermented with yeast to produce ethanol.
This process has several potential advantages. For one, the process is the simplest way to make ethanol from a process point of view – there are fewer steps. One of the by-products of the process, besides ethanol, is the cellulosic “bagasse” that can be burned, gasified, or used in the cellulosic/fermentation process described above. Essentially, almost all of the plant can be used to produce energy.
An interesting aspect of this simpler process is that it might be economically possible for individual farmers to produce ethanol on the farm. One of the challenges is that the collected sugar water is unstable and must be processed fairly quickly after being pressed from the plant.
Cellulosic Ethanol Production
Unlike starch or sugar fermentation, the use of cellulosic plant material to produce sugars for fermentation is more difficult. Lignocellulose is the tough stalk type material that gives plants much of their structural strength. Like starch, cellulose is made up of long chains of sugar (glucose) molecules.
The question becomes: why would one bother with the extra effort required to ferment cellulosic material versus starch? There are at least three key reasons. The first is that cellulosic material is not considered a direct part of the food chain. Another is that there is a great potential for growing cellulosic plants like grasses in marginal agricultural areas. Finally, according to the Argonne National Labs, the amount of fossil fuel need to produce a gallon of cellulosic ethanol is much lower (0.10 gallons) than the amount needed to produce a gallon of corn ethanol (0.76 gallon)5. There are several ways to make ethanol from cellulosic material and two are discussed below.
Enzymatic Hydrolysis
By adding certain enzymes and catalysts to ground cellulose, one can produce the sugars needed for fermentation. Sugars are produced that can be made into ethanol. It should be mentioned at this point that there are no commercial cellulosic ethanol facilities in production.
Gasification-Syngas-Fermentation
This thermochemical process is something of a mix or “hybrid.” The process begins by gasification of organic matter. Typically dry cellulosic plant material (grasses, wood chips, etc.) are used. Gasification is the incomplete burning of carbon-based materials in a high temperature but low oxygen atmosphere. The products of gasification are synthesis (“syn”) gas, heat, tars, and ash. The syngas is composed mainly of carbon monoxide (CO), carbon dioxide (CO2), and hydrogen (H2). The syngas is then cleaned and cooled before being fed into a bioreactor. The gas is bubbled through the bioreactor where microorganisms use the gases as a food source while producing ethanol and other value-added products. The mixture is further processed to separate and recover these products6.
5 Argonne National Labs GREET program.
The diagram below summarizes the two processes described.