Avoiding Antibiotic Residues in Beef Cattle
The documented occurrence of residues of antimicrobial compounds in the tissue of feedlot cattle has been very low. To further reduce the possibility of residues occurring in feedlot cattle, management must be aware that residues may occur in some classes of cattle even though prescribed withdrawal times have been diligently observed. Feedlot management needs to recognize which classes of cattle have a high potential for tissue residue even when good management practices are followed. The total population of cattle in a modern feedyard may be separated into several different categories based on morbidity and medication protocol after arrival at the feedyard.
The highest risk group of animals is the “feedlot sort” class. Data indicate that animals in this “realizer” category may have conditions that preclude normal feedyard performance. These animals may have genetic deficiencies, hardware disease, respiratory deficiency, pathology of plant origin, kidney and/or liver disorders with drug insult, or suppressed immune system as a result of administration of steroids. The live animal swab test (L.A.S.T.) is recommended on all animals of this category. Animals with diseases or abnormal tissues may not metabolize and/or execute antimicrobial residues normally. The L.A.S.T. (urine test) is the complementary part of the swab test on premises (S.T.O.P.) (tissue test) used by packing plant inspectors.
Emergency realizers are a small but significant group of animals. This category includes those with fractures, prolapse, distocia, hard breathers, and other conditions which may necessitate a rather immediate decision to salvage. Medication records will indicate if (1) the animal has been treated in the feedyard, (2) the specified withdrawal times are met, and (3) if the L.A.S.T. is indicated from medical history.
The next class of animals is the “buyer sort” group. These animals may not fit with the pen as a whole for a variety of reasons, one of which may be past medical history. These animals will be merchandised with the pen, but on a grade and yield basis. Medication records should indicate medical history such as diagnosis, date of treatment, and withdrawal times required. If animals in this category were treated, testing may be indicated even if normal withdrawal times have been observed.
Pens of cattle in which “wrecks” have occurred represent another category. These are cattle that may have been highly stressed by the environment to which they were subjected. In addition, stress may have been added by antibiotic or steroid insult prior to shipment. These animals may have been mass-medicated as an immediate preventive measure on arrival at the feedyard. These pens normally have an inordinately high percentage of “pulls” in addition to being subjected to the mass-medication. Medication records should indicate if testing for residues is needed.
The last class of animals are pens of cattle that are received and go on feed with a minimum of problems. However, in this class, some animals may be pulled, treated, recover, and return to the home pen during the “front end” of the feeding period. With approved antimicrobial therapy, these animals should be free of residue at the end of the normal feeding period (at least 100 days from last treatment). Caution should be observed when prescription or extra-label products are used to insure that an appropriate veterinary-client-patient1 (VCP) relationship exists and adequate extended withdrawal time has elapsed.
Some pens of cattle receive no medication at the feedlot. Animals which have not been treated at the feedlot should not have any residues unless they received antimicrobial therapy before arrival at the feedlot.
The prescription for the avoidance of antibiotic residues in feedlot animals is basic and straightforward.
- First and foremost is a “check and balance” record system.
- When using drugs according to label directions, strict adherence to label instruction is essential.
- When using extra label dosages or extra label drugs on the prescription of the consulting veterinarian, a VCP relationship must exist, and extended withdrawal times as indicated by the veterinarian must be adhered to.
- As a final tool, incorporate the Live Animal Swab Test into the normal program of management, whereby the cattle in the “realizer” category are screened for antibiotic residues prior to release from the feedyard.
Label withdrawal times are developed in accordance with Food and Drug Administration guidelines in “normal” animals. Realizer animals are not “normal” animals. Animals suffering from impaired liver or kidney function may have substantially different antibiotic clearance times than those observed in “normal” animals. Consequently, testing the urine of realizer animals with the L.A.S.T. prior to slaughter will enable a feedlot manager to be assured that the animals sent to slaughter will be free of antibiotic residues.
As a method if implementing the check and balance system, animals from all the aforementioned categories may be periodically checked with the L.A.S.T. to confirm the accuracy of the medical record system. Data show that a negative L.A.S.T. indicates freedom from detectable antibiotic residues.
By providing a live animals test for drug residues, the L.A.S.T. is another powerful tool in a check and balance system to provide residue-free food products. After the animal has been treated, has recovered, and the withdrawal times have been exceeded, the L.A.S.T. can assure that drug residues will not be carried to the food chain. In the event that a positive L.A.S.T. is found, the cattleman should withhold the animal from slaughter until the test is negative.
1An appropriate Veterinarian-Client-Patient (VCP) relationship will exist when: (1) the veterinarian has assumed the responsibility for making medical judgments regarding the health of the animal(s) and the need for medical treatment, and the client (owner or other caretaker) has agreed to follow the instructions of the veterinarian; and when (2) there is sufficient knowledge of the animal(s) by the veterinarian to initiate at least a general or preliminary diagnosis of the medical condition of the animal(s). This means that the veterinarian has recently seen and is personally acquainted with the keeping and car of the animal(s) by virtue of an examination of the animal(s), and/or by medically appropriate and timely visits to the premises where the animal(s) are kept; and when (3) the practicing veterinarian is readily available for follow-up in case of adverse reactions or failure of the regimen of therapy. (American Association of Bovine Practitioners Newsletter, March, 1984).
Sample Collection, Care and Use of the Biological Test (L.A.S.T.)
The collection of urine samples for the L.A.S.T. is discussed in detail in USDA publication #601. There are two practical methods of urine sample collection:
- Use of a sterile swab which need only be wet.
- Use of a specimen cup to collect several cc for present and future use.
The sterile swab technique is usually the method of choice. In the normal course of events, if the collector is ready with a swab when the animals enters the chute, the few drops of urine may be obtained rather quickly from both steers and heifers. If the animal does not cooperate, a steer may be made to urinate by massaging the sheath and a heifer will usually urinate if the area just below the vulva is repeatedly stroked.
In the event these techniques fail to produce a sample, the swab may be inserted into the prepuce of the steer and rubber banded to the hair. In the case of the heifer, the swab may be placed in the vulva and secured with a rubber band. The animal may then either be released from the chute and returned to the working area, or merely left to stand in the chute while the collector takes a break. Under most conditions, when the animal is recycled through the working chute, a wet swab will be obtained.
After a urine sample is obtained, the swab is returned to the sterile wrapper, identified, and placed in a clean plastic bag. The procedure serves several purposes. Only a few drops of urine are required to run a valid L.A.S.T. In the event the swab is saturated with excess urine, placing the swab back into the sterile paper wrapper will allow excess urine to be absorbed by the paper. However, in the event the quantity is maintained in the plastic bag technique also solves the problem of having to plate the swabs as they are collected.
Cleanliness is of the utmost importance in performing biological tests. Preventing contamination is especially important when dealing with the steer. If the animal should lie down on a spot where a recently treated steer urinated, contamination may occur. The L.A.S.T. will detect antimicrobial compounds in the parts per billion range; consequently, cleanliness must be emphasized as hand contamination is a major potential problem. Care must be exercised to wash the hand between collections. Use of the plastic bag also isolates the swab sample and helps assure that the opportunity for sample contamination will be reduced.
It may seem monotonous to wash one’s hands so often; however, the procedure is also necessary when plating the swab samples. There are two reasons for a positive result: (1) either the animal is carrying detectable levels of antimicrobial compounds, or (2) the sample was contaminated with an antimicrobial compound.
If a specimen cup is used to collect a significant volume of urine for current or future use, storage facilities are a necessity. The L.A.S.T. is especially reliable, and consistent results may be obtained from samples which have been repeatedly frozen and thawed, or stored at environmental temperatures. However, temperature abuse of a sample should be held to a minimum. This is especially true concerning warm temperatures, where biological degradation is accelerated even with exceptionally stable compounds.
As indicated, L.A.S.T. and S.T.O.P. are biological tests. Both these test utilize the same bacterial organism, culture media, and control disc. As is evident, the tests are essentially identical and consequently complement each other extremely well.
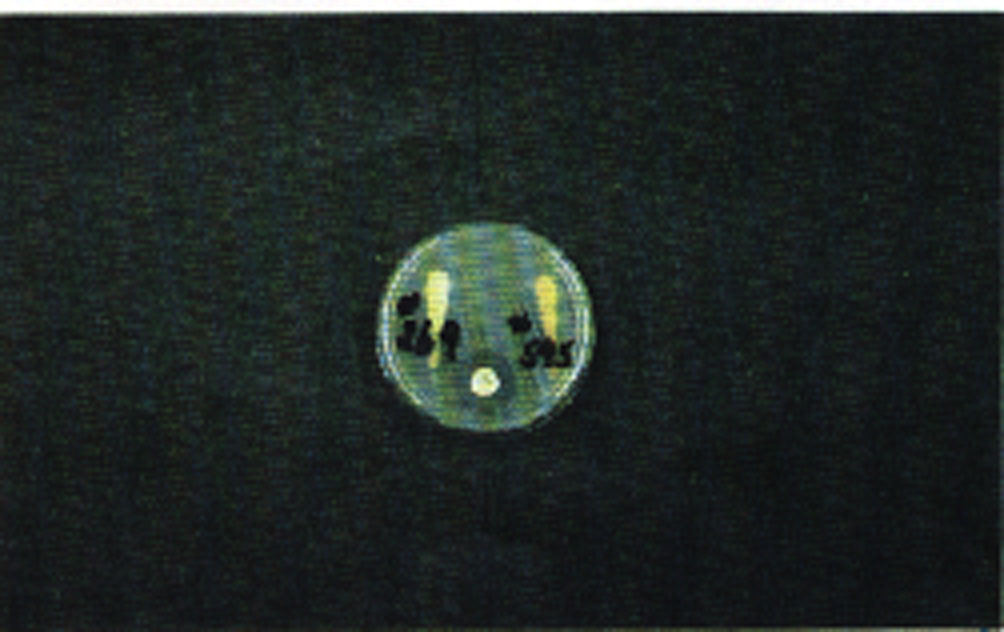
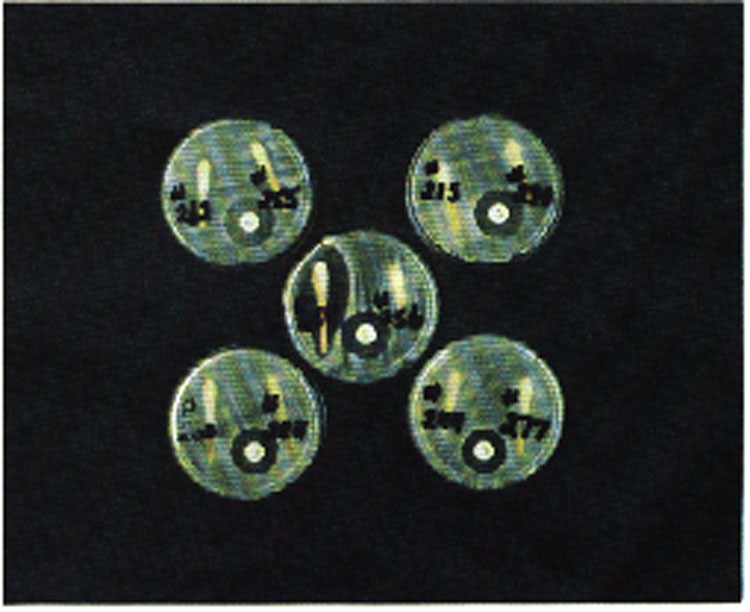
The bacterial organism used is Bacillus subtilus and the mode of action is such that antimicrobial compounds present in excess of certain minimum concentrations inhibit bacterial growth on the culture media. The purpose of the N5 disc (control disc containing 5 micrograms neomycin) is to provide a control which indicates whether or not the test is working. The 5 mcg neomycin disc will produce a remarkably consistent zone of inhibition of 16 mm; no bacterial growth occurs in this zone.
The effectiveness and usefulness of the L.A.S.T. stems from the fact that approved antibiotic compounds are cleared from the body in a rather predictable pattern. Antibiotics are transported throughout the body via the blood stream and are frequently eliminated by the kidney and excreted in a more concentrated form in the urine. Thus, testing urine for the presence of antimicrobial residues is the most reliable means of insuring that detectable tissue residues have cleared the animal.
Use of drugs in cocktail mixtures, high dosage rates, unapproved route of administration, or in a species which does not have approval can only be prescribed by a veterinarian when the Veterinarian-Client-Patient relationship exists. The reason for this is that these products may not follow a predictable pattern of elimination, be retained in the body for an extended time, or damage the organs that break down and eliminate these products. This tissue damage could cause the animal to become a chronic or a realizer. Extended withdrawal periods and a L.A.S.T. may be indicated on these cattle prior to shipment.
How to Conduct the Live Animal Swab Test for Antimicrobial Residues
Items needed to perform the L.A.S.T. are (1) open bottom chute, (2) incubator, (3) thermometer, (4) container for water, (5) sterile laboratory swabs and/or specimen cups, (6) small forceps, (7) N5 control discs (5 micrograms neomycin), (8) Bacillus subtilus suspension (bacterial suspended in liquid), (9) felt tip marker to I.D. specimens, and (10) “baggies” in which to place the wet swab and sheath for carrying to the lab area to prevent excessive drying and contamination. Cost of the test is normal, ranging from $.40 to $2.00 per plate. Two tests may be performed per plate, thereby reducing the cost. The greatest variables in cost are the quantity purchased and the supplier.
L.A.S.T. plates may be purchased packaged in individually sealed plastic strips or in units of 10 per package. The plates all contain pre-poured culture media. In the event only one plate is to be used, care must be taken to insure that seals are not broken on individually packaged plates. All plates should be refrigerated to prevent dehydration of the culture media. Storing the plates bottom up also aids in maintaining the integrity of the culture media. DO NOT FREEZE.
After removing the plate from its unit, inoculate the media with the Bacillus subtilus organisms. A sterile swab may be dipped into a vial or organisms, the agar is streaked in a back and forth manner starting from top to bottom, then rotated 90° and the process repeated. This procedure is illustrated in USDA publication #601.
Following streaking, a N5 (5 mcg Neomycin) control disc is placed in the agar using forceps or a dispenser. Seat the disc on the agar where it falls. The control disc produces a 16 mm clear zone of inhibition and indicates whether or not the test is working.
Two test may be run per plate. Following inoculation, the animal I.D. numbers are written on the bottom of the plate. The appropriate swabs are then broken off and placed over the appropriate numbers and lightly pressed onto the agar, exercising care not to break the agar surface. The process is now complete and the plate may be placed in an oven preheated to 85°F (29.5°C), with the bottom of the plate (numbered side) up, and allowed to incubate for 12 hours. Interpretation of the results are graphically explained in the USDA publication #601, as well as in Figures 1 and 2.
The incubators may be as expensive or inexpensive as one wishes. Also, the 85°F temperature is not absolute. The acceptable temperature range is 82°-86°F (28°-30°C) and aids the use of homemade incubators.
The L.A.S.T. is an inexpensive and accurate tool for determining the presence of detectable antimicrobial residues in live beef cattle. This tool, coupled with an accurate record keeping system, may be used to assure the beef producer, consumer, USDA Inspector, and the FDA regulatory agency that U.S. produced beef is, in fact, a wholesome product.
Minimum guidelines on drug withdrawal times are as follows:
- For label dosages, use label directions for withdrawal.
- For any extra label dosage of approved drugs, a V-C-P relationship must exist as a guide for withdrawal times. Withdrawal times must be increased in proportion to the increase in dosage.
- For extra label dosage of drugs not approved for beef cattle, follow the guidelines for withdrawal established by the prescribing veterinarian.
- Neomycin has no safe established withdrawal period when injected.
The Food and Drug Administration has approved over 1000 drugs, both over the counter and veterinary prescription, for use in food animals. These products save producers and consumers millions of dollars each year in production costs. Always read the label and follow all directions for dosage, route of administration, animal to be used with, and withdrawal period. Table 1 may help the producer understand withdrawal times of drugs before slaughter.
Figure 1. Both tests are negative for detectable antibiotic residues.
Figure 2. The center positive swab indicates antibiotic residue after the observance of published withdrawal time.
Table 1. Beef Cattle Drug List.
-these drugs are antimicrobial products
*withdrawal days varies with brand names and/or drug concentration