Understanding your Lawn and Garden Soil Test
Soil testing is a critical component for a successful lawn and garden. With so many variables affecting plant performance including weather, pest and disease pressure, it is important to provide plants with appropriate nutrients that will make them healthier and more resilient to the natural challenges they face. Regardless of the location (lawn, vegetable garden or ornamental landscape), a soil test will provide gardeners and lawn care providers with the basic information on how to maintain and improve the overall nutrient balance for the plants. Soil testing for lawns and gardens recently has gained popularity in Oklahoma. This fact sheet aims to help homeowners and lawn care professionals understand their soil test reports.
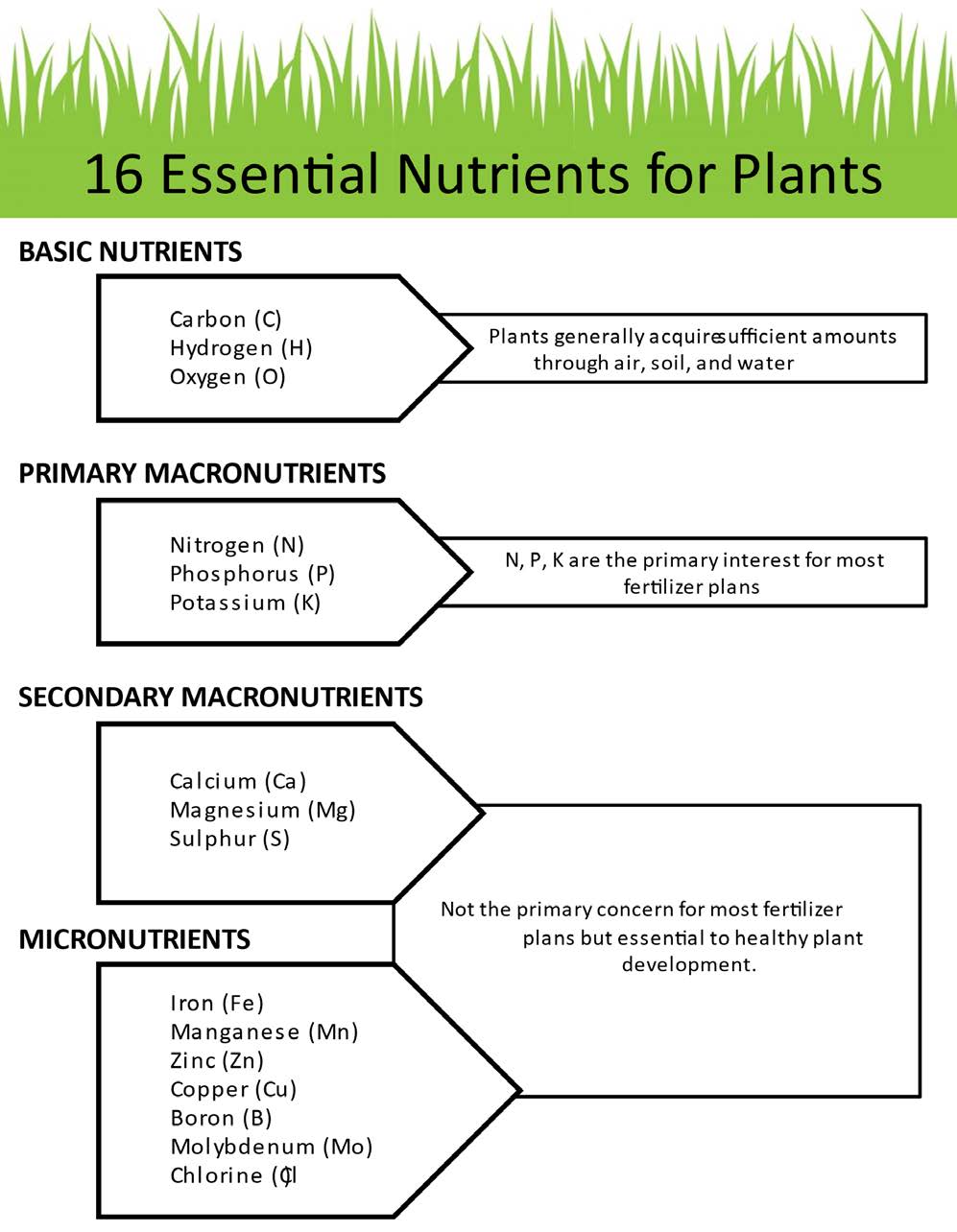
There are 16 essential nutrients to support a plant’s function and they are needed in different quantities (See Figure 1), although some scientists think nickel (Ni) and silica (Si) are beneficial. Only a few nutrients need to be supplied periodically and others are provided by soil, water and air. Nitrogen (N), phosphorus (P) and potassium (K) are needed in large quantities and the soil often does not supply the adequate amount. For this reason, these three nutrients are present in most complete fertilizer products and are shown on the bag as percentages by weight. The relative percentages of nutrients in the fertilizer are always shown in the order of N, P and K expressed as N%, P2O5% and K2O%, respectively, such as 10-20-10 as seen in Figure 2. Therefore, plant available N, P and K levels are typically included in a routine soil test package (Figure 3). A soil sample determines whether the soil has appropriate amounts of N, P and K, and others (if requested) for a particular crop. For more information about soil nutrient and pH level recommendations for lawn, gardens and horticulture crops, see Oklahoma Homeowner’s Handbook for Soil and Nutrient Management and OSU Extension fact sheet Soil Test Interpretations for Vegetable Crops (HLA-6036).
Figure 1: The 16 essential nutrients are categorized based on the quantity needed by the plant.
Nitrogen
Nitrogen is the main component in chlorophyll, all amino acids, proteins and often is associated with healthy, green vegetation. Turfgrass fertilizers typically have a higher nitrogen percentage than others to support a green lawn. If grass clippings are left on the yard using a mulching lawn mower, they will decompose and return most of the nitrogen and other nutrients back into the soil. This and life extension of landfills by reducing landscape waste is why the “Don’t Bag It” cultural management system was introduced. See Recycling Yard Waste: “Don’t Bag It” Lawn Care Plan (L-253) for more information regarding benefits of recycling lawn clippings.
Nitrogen is considered a mobile nutrient, meaning that it will readily dissolve and move through the soil profile with water. Therefore, water (e.g., irrigation or rainfall) can leach nitrogen below the rooting zone of many plants, often making nitrogen the most deficient nutrient for plants.
Figure 2. All fertilizer labels will have three numbers representing the actual nitrogen, phosphate and potassium content by weight. This first fertilizer bag in the picture has 13% nitrogen, 13% phosphate (P2O5) and 13% potassium (K2O), while the second one has 29%, 3% and 4% of those nutrients, respectively. Nutrient recommendations on a soil test report are in pounds of nitrogen, P2O5 and K2O, so no conversion is needed.
Phosphorus and Potassium
Phosphorus and potassium also are essential to a plant’s functions necessary for survival. Phosphorus helps a plant grow by assisting with early root formation. Because it also stimulates flower and seed production, fertilizers for flowers and vegetables often have a larger second number on the product label, meaning it contains more phosphorus. Potassium aids the plant’s overall health and disease resistance, and plays a role in the ripening of fruit.
The Mehlich 3 extraction method has been used for plant-available phosphorus and potassium analysis in Oklahoma and many other central and eastern states. The estimated availability of the nutrients is reported as an index and percent sufficiency in the soil. A soil test phosphorus (STP) index of 65 and soil test potassium (STK) index of 300 represents 100% sufficiency, which means there is no need to apply additional phosphorus or potassium once the STP or STK reaches 65 or 300, respectively. Phosphorus and potassium interact differently with the soil than nitrogen and are considered less mobile in the soil. The soil test phosphorus is less subject to change (less rapid of change) than potassium levels and potassium levels less subject to change than nitrogen levels through time. Phosphorus and potassium are typically applied once per season if needed, while nitrogen needs to be applied several times per growing season to minimize leaching loss. The amount of nitrogen recommended on soil test reports is for each application, and the number of applications per season depends on the plant and purpose. In the case of very sandy soils that are subject to extremes of leaching, nitrogen, phosphorus and potassium may be administered in smaller quantities, more frequently and throughout the year.
Why More is Not Better
While soil nutrients can vary spatially and temporally and should be regularly tested, often only nitrogen is needed in lawn and garden situations, especially on heavy-textured soils (those with low sand content), due to low nutrient leaching losses and past phosphorus and potassium applications (see CR-6467 Soil Test Summary for Oklahoma Lawn and Garden for detail). Nitrogen is like gasoline in a car, which regularly needs to be filled up. Phosphorus and potassium are like the oil in the car, which only needs to be added occasionally as the dipstick indicates. With this concept, it would be wasteful and unnecessary to add engine oil every time gas is added. However, that is often what gardeners are doing when they regularly apply a complete fertilizer (contains all three nutrients) season after season without a soil test. An overabundance of these nutrients is not only an unnecessary expense, but it might become a pollutant.
Typically, plants need more nitrogen than phosphorus. When a complete fertilizer is applied season after season, the soil profile can become overwhelmed with some nutrients, especially phosphorus. Like a faucet that drips slowly into a bowl, it will eventually overflow. Although most yards are relatively small in area, collectively, this can become a major problem in neighborhoods and regions as excess nitrogen and phosphorus flow off yards and down storm drains, ending up in retention ponds, streams and larger bodies of water. The increased load of nutrients into water sets off a chain reaction of disruption in the water’s ecosystem by causing harmful algae blooms that deplete oxygen levels in the water, leading to fish kills and other negative impacts on water quality. This process is called eutrophication. Nitrogen and phosphorus are the two main nutrients for this nonpoint source pollution and explain why more is not always better when it comes to fertilizer.
What is pH?
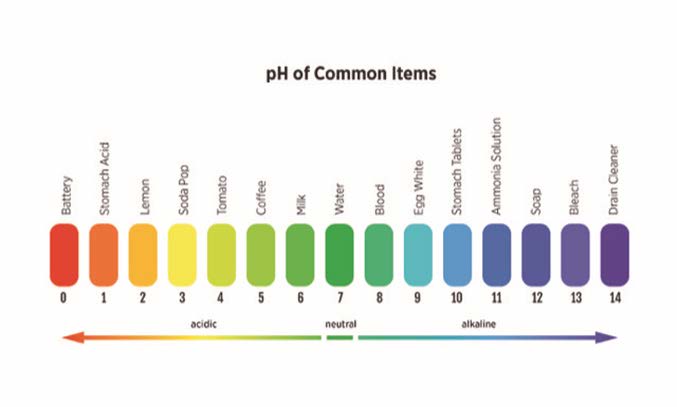
In addition to nitrogen, phosphorus and potassium levels, a routine soil test also will provide the soil pH (the measurement of soil acidity or alkalinity). The pH is expressed on a scale from 0 to 14. Pure water has a pH of 7, which is considered neutral. Numbers lower than 7 is considered acidic; while numbers higher than 7 represent a basic condition. Many household goods are either acidic or basic, as shown in Figure 4. While most people know that pH references whether something is an acid (acidic) or a base (alkaline), it also is important to understand why the soil’s pH is important.
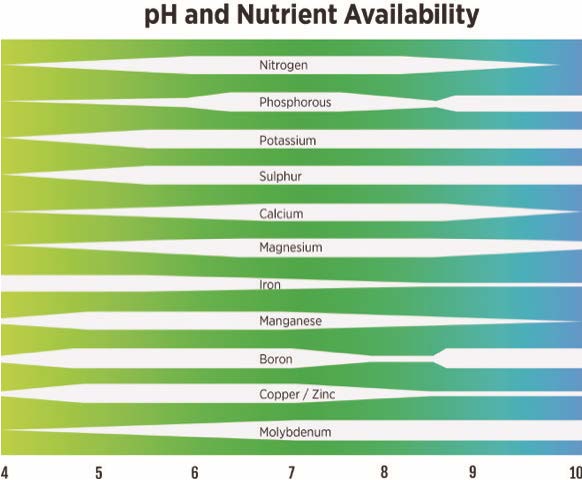
The soil’s pH is an index that provides a better understanding of the soil environment in which the plants are growing (or not growing in some cases). Soil pH not only affects plant performance directly, but also impacts nutrient availability. Figure 5 shows the availability of elements at various pH levels. At a low or high pH, a plant may have a nutrient deficiency, when in fact that element is in the soil, but due to the pH the nutrient is not available for the plant to access. Iron chlorosis in pin oaks, as seen in Figure 6, is a common problem in Oklahoma due to alkaline soils, which makes iron less available. Knowing the pH value will help identify what nutrients are available to a plant. Most plants prefer a neutral and slightly acidic soil with a pH range of 6.0 to 7.5 where most nutrients are more available. However, some plants, such as blueberries and azaleas, prefer a more acidic soil with a pH of 4.5 to 5.5. Fescue can tolerant pH as low as 4.5, but bermudagrass prefers pH above 5.6.
There are various natural factors that influence a soil’s pH, including the parent rock material where the soil is formed and rainfall. Soil pH tends to be more acidic in eastern Oklahoma where the average annual rainfall is 40 inches to 56 inches and alkaline or calcareous in western Oklahoma, which has an average annual rainfall of 15 inches to 30 inches.
The repetitive application of nitrogen fertilizer also can gradually acidify soils. This is especially true where a large amount of plant biomass has been removed from a site, such as the gathering of crop yield, regular removal of turfgrass clippings or large-scale wood lot harvest has occurred. This can be a problem on lawns that are regularly applied with ammoniacal nitrogen and removal of clippings. Gardeners may think they are doing everything correctly and expect the same beautiful lawn, but instead have a lawn that is not doing well. Testing the soil pH will quickly identify if this is the problem. Lime is generally recommended if the soil pH is below the ideal range for a particular plant. The soil pH determines if lime is needed or not, while the buffer index determines the amount of lime needed. If the soil pH is above the ideal range, recommendation for acidification is not offered on the report since it is not easy to lower soil pH and, with the exception of acidic soil-loving plants, most plants can tolerant a wide range of pH. For acid-loving plants such as blueberry and azalea, elemental sulfur is used to lower soil pH. See Oklahoma Homeowner’s Handbook for Soil and Nutrient Management for details on sulfur amendment.
A routine soil test is a straight-forward process that will provide information on
the nitrogen, phosphorus and potassium levels as well as the soil pH value. Collecting
the
sample will take about 20 minutes and is even easy for a novice gardener. While the
steps are simple, the quality of the sample affects the accuracy of the test. To collect
a good soil sample, see L-249 Soil Testing the Right First Step.
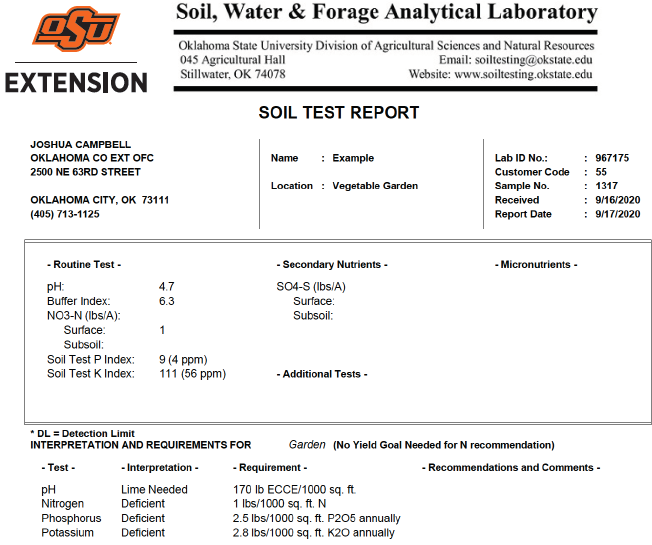
Figure 3. A typical soil test report showing all three main nutrients are deficient and pH is too low. County Extension educators can assist with additional recommendations on specific products available locally to supply those nutrients and/or lime. ECCE stands for effective calcium carbonate effectiveness, which is available on the product label.
Figure 4. The pH scale of 0 to 14 is used to identify aqueous solutions as being acidic or alkaline. This graphic shows many of our household items pH values with pure water is defined as a neutral pH of 7. Graphic by Vince Giannotti.
Figure 5. Nutrients availability affected by pH levels. Most plants prefer a pH of 6.0 to 7.5 where many of these nutrients are most available. Graphic by Vince Giannotti.
Figure 6. Iron chlorosis symptoms exhibit yellowing between the leaf veins. In pin oaks, it can occur on a few leaves, branches or the whole tree. In severe cases, it can cause the leaves to scorch. Persistent iron deficiency can lead to the tree’s decline. Photo credit: L. Joseph O’Brien, USDA Forest Service, Bugwood.org
Additional Resources:
- Soil Test Interpretations for Vegetable Crops, HLA-6036
- Oklahoma Homeowner’s Handbook for Soil and Nutrient Management, E-1003
- Recycling Yard Waste: “Don’t Bag It” Lawn Care Plan, L-253
- Improving Garden Soil Fertility, HLA-6007
- Responsible Lawn Care: Protection for Your Creek or Lake, L-346
- How to get a Good Soil Sample, PSS-2207
Videos:
- How to Collect Soil Samples in your Area
- Understanding Soil pH
- Soil Test: Interpreting the Report
- Calculating the Correct Application Rate for Garden Fertilizers
- Fertilizer Application of Organic Fertilizers