Soilborne Blight Diseases of Peanut
Southern blight and Sclerotinia blight are the two most important soilborne diseases of peanuts in Oklahoma. These diseases have the potential to cause severe economic losses, because they cause rapid wilting and death of peanut plants prior to maturity. The disease cycles and symptom progression of the two diseases are similar, but the fungicides and many of the cultural practices used to manage them are different. Sclerotinia blight can also be potentially more destructive, persistent, and difficult to control than southern blight. Therefore, it is critical that each disease be properly identified so that appropriate controls can be implemented.
Southern Blight (Sclerotium rolfsii)
Southern blight, also known as stem rot, is caused by a soilborne fungus. The disease is widespread on peanuts and other crops in Oklahoma. Disease development is favored by hot and humid conditions in the peanut canopy. Therefore, the disease occurs mainly in mid- to late-season in Oklahoma. The fungus primarily attacks the base of stems near the soil line, but any plant part in contact with soil may be damaged. Infected plants are generally killed prior to maturity. Peg and pod infections are common and result in pod loss at harvest. Populations of S. rolfsii increase in infested fields cropped to peanut unless control measures are taken. High populations of the pathogen combined with favorable conditions for southern blight can result in yield losses of 25 percent or more.
Symptoms
The first readily apparent symptom of southern blight is rapid yellowing and wilting of limbs or entire plants (Figure 1). Affected limbs and plants then turn brown and die as a result of decay of the lower stem. Advanced symptoms of southern blight are very similar to those of Sclerotinia blight. The base of diseased plants must be closely examined for signs of the fungus to distinguish between the two diseases. Southern blight is characterized by a white moldy growth (mycelium) covering lower stems and imparting a white-washed appearance to the base of affected plants (Figure 2). The mycelium of the southern blight fungus is coarse, rope-like, closely appressed to stems, and may radiate out over the soil surface (Figure 3). This is best observed when the soil surface is moist. Mycelium may also be seen growing on decaying plant matter on the soil surface. Lesions (dead areas) on infected stems and pegs are at first light brown, and then become dark brown. Advanced symptoms develop when lesions coalesce to girdle the lower stem. Small, round, mustard seed-like structures soon develop on the mycelium (Figure 4). These structures are called sclerotia and serve as reproductive and survival structures of the fungus. The sclerotia first appear as white tufts that later become light brown and finally dark brown at maturity. Sclerotia are uniformly round, about 1/16 inch in diameter, and may be produced in large quantities on dead plant parts.

Figure 1. Wilted plant or “flagging” from either southern blight or Sclerotinia blight. The base of this plant must be closely examined to determine which disease is the cause.

Figure 2. Mycelium of the southern blight fungus at the base of an infected plant. Note the sclerotia developing on the leaf litter.
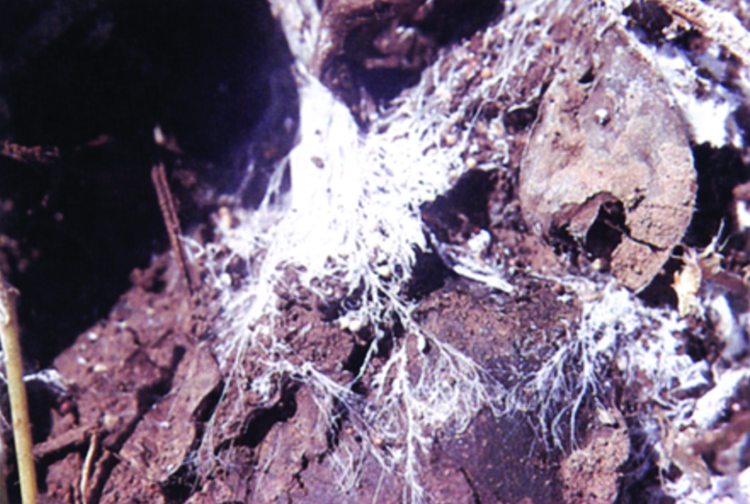
Figure 3. Coarse, rope-like mycelium of the southern blight fungus radiating out over the surface of moist soil.

Figure 4. Close-up of the small, uniformly round sclerotia of the southern blight fungus.
Disease Cycle
Initial southern blight infections arise from sclerotia, which overwinter in peanut fields. Sclerotia of the fungus are similar to seeds of a plant because they remain dormant until conditions become favorable for germination. Conditions that favor germination and infection are high moisture, warm to hot temperatures, and the presence of a susceptible plant. Moisture requirements can be met from irrigation or rainfall. Conditions that favor growth of the fungus normally begin to develop at pegging when limbs grow rapidly along the soil surface and the canopy becomes dense. Organic matter is thought to stimulate the germination of sclerotia and serve as an initial food base for the fungus. The fungus then grows outward from the food sources and infects plant parts in contact with the soil surface. An acid produced by the fungus kills plant tissues. Disease development below the soil surface can be extensive in sandy, well-aerated soils, resulting in severe peg and pod rot.
As the disease progresses, numerous sclerotia develop that easily dislodge and fall to the soil surface. Sclerotia survive well at the soil surface but survive poorly when buried deeply because the fungus has a high demand for oxygen. Sclerotia at or near the soil surface can survive three to four years, while survival is a year or less when sclerotia are buried deeply.
The southern blight fungus does not produce spores that move in the air, so the disease is confined to localized areas in a field where sclerotia reside. Disease levels are typically high where a large number of sclerotia reside near the soil surface and conditions are favorable for southern blight.
Control
Cultural practices to manage southern blight can be very effective, because actions taken to reduce the populations of sclerotia at the soil surface can directly reduce disease incidence. Integration of cultural practices with a fungicide program will generally result in the best control.
Soil tillage in between crops is beneficial for burying sclerotia and reducing levels of disease compared to no tillage. Deep tillage with a moldboard plow is most effective, but this practice is rarely employed because of soil conservation and economic concerns. Cultivation during the growing season should be avoided because it encourages southern blight development. Increased soil aeration from cultivation allows deeper penetration of the fungus where pegs and pods can be readily attacked. Cultivation known as dirting, which moves soil up against plants should be avoided. Buried sclerotia are brought to the soil surface by this practice and deposited near the crown area causing increased levels of disease. Irrigation should be properly timed to allow application of adequate but not excessive amounts of water and to maximize the dry period between irrigations. Frequent application of small amounts of water should be avoided as prolonged periods of canopy wetness favor disease development.
Crop rotation is an effective practice for management of southern blight, but rotation crops must be carefully selected because the southern blight fungus has a wide host range. Corn, grain sorghum, sudan grass, and cotton are excellent rotation crops for southern blight control. Two-year rotations are required where southern blight has become severe. Rotations with susceptible crops such as soybeans, alfalfa, peppers, and melons should be avoided. Fallowing between peanut crops is only effective if land is kept free of weeds.
A fungicide program is beneficial for fields where southern blight is a known problem. Several fungicides are registered for use on peanuts that provide good control. Most of these fungicides also control leaf spots, which makes their use economical. Fungicides are generally recommended as blocks of three to four mid-season sprays on 14-day intervals, or as two mid-season applications on four-week intervals.
The best results are achieved when the fungicide program is started before symptoms appear. The optimal starting point is at row closure,about 60 to 70 days after planting. Alternatively, fungicide application may be delayed until the disease first appears. Reduced costs may result from delaying the first application so fewer applications per season are made. However, the reduced cost may be offset by a reduction in the level of control achieved. After a fungicide application, allow the fungicide to dry on foliage to protect against leaf spot. Then insure that the remaining fungicide is redistributed to the lower stems by rain or irrigation within four days after application. Fields should be scouted weekly throughout the season to monitor the status of southern blight and other diseases. Currently registered fungicides can increase yields by more than 1,000 lb/A where southern blight develops to severe levels. Consult the most recent Peanut Production Guide (Extension Circular E-608) or OSU Extension Agents Handbook of Insect, Plant Disease, and Weed Control (Extension Circular E-832) available at you local extension office for the latest information on fungicides for control of southern blight. Unfortunately, most fungicides registered for southern blight are not effective on Sclerotinia blight.
Southern Blight Management Tips
- Practice crop rotation with non-susceptible crops such as corn, sorghum, or cotton.
- Use tillage in between peanut crops to bury sclerotia and crop residue.
- Avoid cultivation during the peanut cropping period, particularly the practice of dirting peanuts where soil is moved against plants during cultivation.
- Use a fungicide program to reduce losses where southern blight is a known problem.
- Time irrigations to apply adequate but not excessive amounts of water and avoid frequently applying small amounts of water.
Sclerotinia Blight (Sclerotinia minor)
Sclerotinia blight, caused by a soilborne fungus is a destructive mid- to late-season disease of peanuts. The disease is widespread in Oklahoma and in Texas. Sclerotinia blight is similar to southern blight in that the fungus survives in the soil by forming resistant seed-like structures (sclerotia) and attacks plants near the soil line. Sclerotinia blight, however, is potentially more damaging because of its ability to rapidly spread within the peanut canopy. Yield losses in susceptible varieties typically exceed 50 percent in experimental plots infested with the fungus The disease usually becomes severe late in the growing season, but may occur in Oklahoma as early as July, depending upon weather conditions. Because the disease often occurs late in crop development, yield reductions are primarily from stem and peg rot that cause pod loss during digging.
Symptoms
Symptoms of Sclerotinia blight may appear anytime from mid- to late- season when weather conditions are cool to moderately warm and wet. The first symptom of Sclerotinia blight is the wilting and yellowing of lateral or main branches (Figure 1). Affected branches near the soil line must be closely examined for definitive identification of Sclerotinia. A fluffy or cottony, white moldy growth (mycelium) develops around diseased areas of lower stems (Figure 5). The mycelium of Sclerotinia is most easily observed in mornings when dew is present or after a rain or irrigation and may disappear later in the day when the lower canopy is dry. This is in contrast with the coarse, rope-like mycelium of southern blight, which generally remains visible under drier conditions. Dead areas (lesions) develop on affected stems that appear elongated and light tan to white in color (Figure 6). Soon entire limbs wilt and turn pale green. Affected branches die and turn dark brown in color and eventually whole plants may be killed.

Figure 5. White, fluffy mycelium of Sclerotinia growing on infected stems in a wet canopy.

Figure 6. Vines killed by Sclerotinia blight are pale white or straw-colored.
The small seed-like survival structures (sclerotia) of the fungus are black and irregularly shaped, ranging in size from 1/16 to 1/8 inch (Figure 7). These sclerotia may aggregate into larger masses that appear similar to mouse droppings. These are clearly different from the tan to brown and perfectly round sclerotia of the southern blight fungus. Sclerotia may not be evident during the early stages of disease development. Sclerotia are formed in and on infected stems, leaflets, pegs, pods, and roots.

Figure 7. Small, irregularly shaped, black sclerotia of Sclerotinia.
Disease Cycle
Sclerotinia overwinters and survives between peanut crops in the soil as sclerotia. Viable sclerotia, capable of infecting peanuts, have been found in the plow layer of infested soils for up to four years after a peanut crop. The disease has appeared in peanut fields left out of production for more than five years suggesting that sclerotia may persist in soil even longer. Sclerotia rapidly germinate and infect plants under conditions of cool to moderate average daily temperatures (65 to 70 F), high soil moisture, and high relative humidity (95 to 100 percent). Stems, leaves, and pegs lying adjacent to germinating sclerotia become infected. The disease can spread rapidly under favorable conditions as infections can occur in 24 hours and symptoms appear in 72 hours. The fungus directly infects healthy plant parts, but mechanically damaged vines are most prone to infection. Sclerotia are formed in large numbers after the fungus completely decays infected plant parts. Sclerotia become dislodged from plants and fall to the soil or remain in infected plant parts left after harvest.
Plant to plant spread of Sclerotinia blight is limited because the fungus does not produce airborne spores during the growing season. Sclerotia may germinate and form a small, cup-shaped mushroom which produces airborne spores. In Oklahoma, these spores are produced in the early spring, several weeks before peanuts are planted. Spread of the disease within the canopy occurs only by direct contact of healthy and diseased plant parts. Conditions favoring active growth of the fungus also favor spread to healthy plants. Centers of diseased plants occur in fields because of the limited distance which secondary spread occurs. Centers of blighted plants become more numerous as concentrations of sclerotia in soil increase. Sclerotia are also the primary means of long distance spread of Sclerotinia blight.
Sclerotia are easily moved from field to field with soil or infested peanut straw. Movement of farm implements from infested to clean fields is an important means of disease spread. Research in Oklahoma has also shown that sclerotia fed to cattle in infested peanut hay can pass through the digestive tract of cattle and remain viable. Sclerotia may be then deposited with dung in clean fields. Sclerotia are also small and light enough to be moved with wind-driven soil. Water moving over soil infested with Sclerotinia may also move sclerotia within fields or to nearby fields. Contaminated water could arise from irrigation or rainfall runoff from infested fields. Tillage practices help move sclerotia throughout the plow zone, but probably have a minimal effect on increasing the size of contaminated areas. Plowing buries sclerotia at the surface and in turn brings buried sclerotia to the surface. Expansion of infested areas from plowing alone occurs, but at a slow rate.
Sclerotinia is seedborne and has been found as a contaminant of seed from research plots at levels as high as 12%. Transmission of the disease from contaminated seed lots to new plants has been demonstrated in the greenhouse under very favorable conditions, but not in the field.
More than 400 commercial seed lots from different areas of Oklahoma have been tested to date for the presence of Sclerotinia and only four were positive. The level of contamination in those four seed lots averaged only 0.5 percent. This research indicates that although introduction of Sclerotinia into clean fields by seed is possible, it may not be an important means.
Sclerotinia has a wide host range which includes both cultivated and weed hosts.
Susceptible crops include alfalfa, rape, canola, sunflower, bean, sweet potato, tobacco, cole crops, and soybean. However, some of these crops such as soybean may not become severely diseased when grown in infested fields in Oklahoma. Alternate hosts may aid in the establishment of Sclerotinia in clean fields or its build-up in fields already infested.
Control
Because Sclerotinia blight can be very damaging and difficult to control, steps should be taken to limit the spread of the fungus and its entry into clean fields. Tractors, plows, combines, and other farm implements leaving infested fields should be carefully cleaned and washed prior to entering clean fields. Soil and peanut straw should be completely removed from these implements. Cattle fed with peanut hay from fields infested with Sclerotinia should not be pastured on clean fields. Peanut hay taken from infested fields should not be fed to cattle grazing on clean fields. To eliminate the potential for importing the disease into fields on peanut seed, seed should be treated with a fungicide known to reduce Sclerotinia.
A combination of cultural and chemical management practices will provide the most satisfactory control of Sclerotinia blight. Proper irrigation is important. Enough water should be applied at each irrigation to thoroughly wet the root zone and allowing a prolonged drying of the soil surface before the next application. Avoid frequent irrigations with small amounts of water.
Excessive vine damage should be avoided because Sclerotinia easily infects wounded plant parts. Vine injury can be minimized by using tractors with narrow tires.
Variety selection is critical for problem fields. The upright growth habit of Spanish varieties forms a more open canopy than runner varieties which have a dense and prostrate canopy. This open canopy is less favorable to development of Sclerotinia blight. Since the release of Tamspan 90, a resistant Spanish variety, several runner and spanish varieties have been released with moderate levels of resistance. These varieties should be grown where Sclerotinia blight is anticipated. Reductions in disease levels by planting resistant varieties typically exceeds 50 percent and yield responses have ranged to 500 to 1500 lb/A. However, with prolonged periods of cool rainy weather, even resistant varieties may become severely diseased. Consult the most recent Peanut Production Guide (Extension Circular E-608) for the latest information on variety performance and disease resistance.
Another important aspect of variety selection is maturity length. Spanish and other short-season varieties have the advantage of earlier harvest dates. Because Sclerotinia blight is normally most severe in September and October, long-season runner varieties are particularly vulnerable. A short-season variety that matures 20 to 30 days earlier than a long-season variety will reduce losses by lessening the exposure of plants to blight-favorable weather.
A fungicide program is usually required where Sclerotinia is a problem and a susceptible variety is grown. Proper application timing and method of application are essential for maximum disease control. Fungicides should be applied preventively because they protect healthy plant parts from infection and do not cure established infections or kill sclerotia. Therefore, the first fungicide application should be made when symptoms are first noticed, or preferably before, when conditions first become favorable for disease after pegging. Since most fungicide is deposited in the upper plant canopy following ground or aerial application, redistribution to the lower canopy where the disease is active with water shortly after application (within four days) is beneficial. This can be accomplished with rainfall or irrigation. Fields should be carefully scouted throughout the season to monitor the activity of Sclerotinia blight. Consult the most recent Peanut Production Guide (Extension Circular E-608) or OSU Extension Agents Handbook of Insect, Plant Disease, and Weed Control (Extension Circular E-832) available at you local extension office for the latest information on fungicides for control of Sclerotinia blight.
Because sclerotia of Sclerotinia are persistent in soil, it is unlikely that crop rotation will result in satisfactory disease control. Conversely, continuous cropping of susceptible runner varieties will result in a rapid build-up of Sclerotinia blight making profitable peanut production difficult. A carefully selected rotation should significantly reduce the rate of blight increase in infested fields. Corn, sorghum, cotton, and small grains are excellent rotation crops for reducing build-up of Sclerotinia.
Sclerotinia Blight Management Tips
- Avoid spreading Sclerotinia from infested to clean fields on equipment, peanut hay, or animals.
- Plant spanish varieties or a resistant variety in fields with a history of severe Sclerotinia blight.
- Time irrigations to apply adequate but not excessive amounts of water and avoid frequently applying small amounts of water.
- Avoid excessive mechanical injury to vines.
- Apply a fungicide recommended for control of Sclerotinia blight.
References
Melouk, H.A., and F.M. Shokes (eds). 1995. Peanut Health Management. The American Phytopathological Society, St. Paul, MN.
Kokalis-Burelle, N., D. M. Porter, R. Rodríguez-Kábana, D. H. Smith, and P.
Subrahmanyam (eds). 1997. Compendium of Peanut Diseases. The American Phytopathological Society, St. Paul, MN.
John P. Damicone
Extension Plant Pathologist
