Organic Matter Content of Wastewater and Manure
Organic Matter (OM) plays a large role in the environment. The OM content of soil affects nutrient retention, water holding capacity, and the soil’s ability to provide nutrients for plant growth. The OM content of wastewater discharged to a stream determines how much oxygen is available for fish to breathe. This Fact Sheet defines OM in byproduct materials, and shows how the many different measures of OM are used to predict the material’s behavior in the environment.
What is Organic Matter?
To a chemist, an Organic Chemical is any compound that contains Carbon. To a biologist, OM is living material or material that was once alive. Soil Scientists define a special type of OM called humus as, “the all-but-stable fraction of soil OM remaining after the plant and animal residues have decomposed.” Environmental Engineers have an even simpler definition, if it burns at 550 C, it is organic. Running through these definitions is a constant theme. Organic matter is connected to life. And as you will soon see, OM is also connected to energy.
The Connection between Organic Matter and Energy
Simply put, nature stores energy using carbon. Take crops growing in a field. Plants
use energy from sunlight, carbon dioxide (CO2) from the air, and moisture from the soil to create carbohydrates (CH2O) through photosynthesis (Figure 1). Carbohydrates are the building blocks of life.
In other words, they are the basic ingredients of OM.
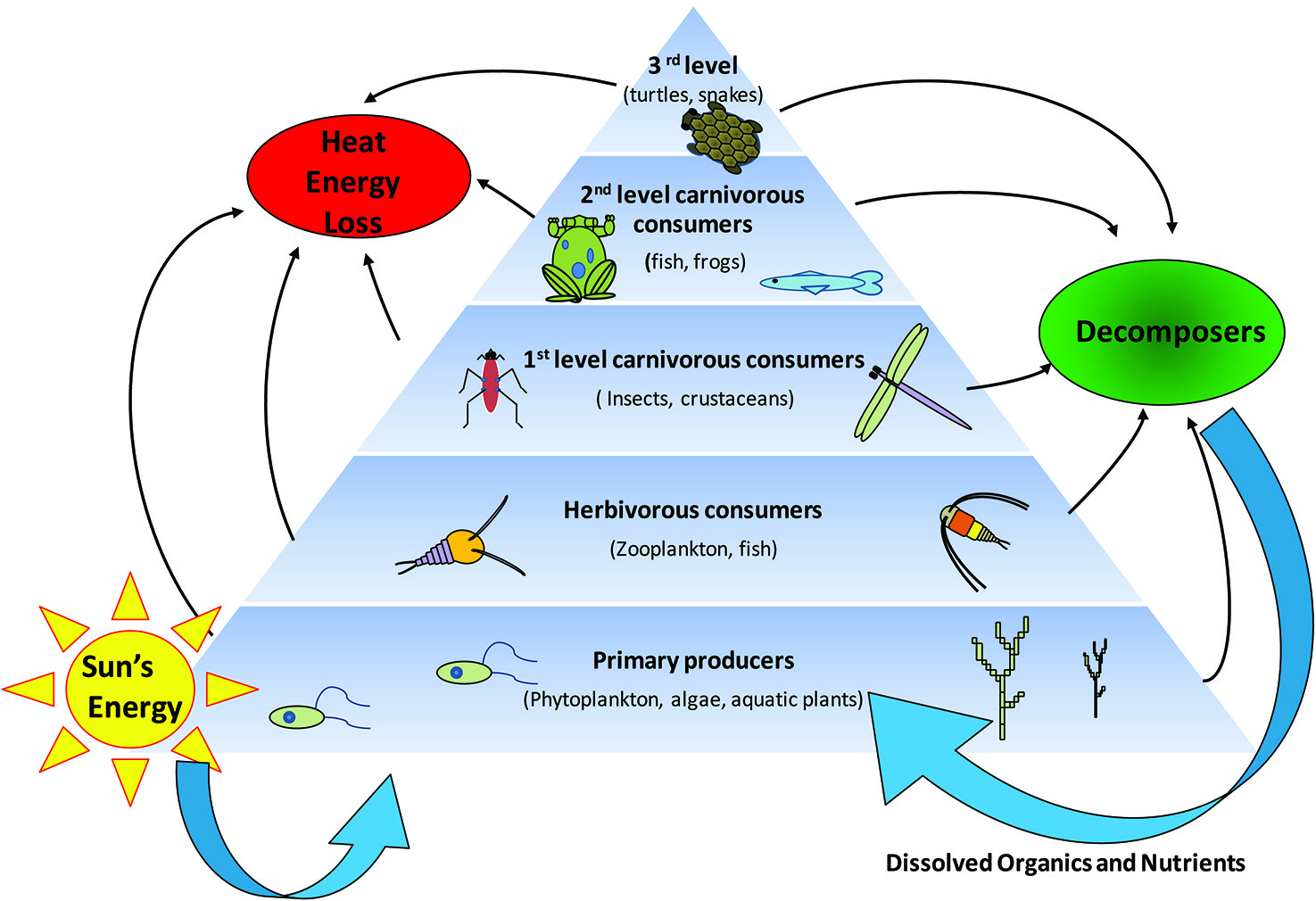
Conversion of energy to organic matter, sometimes called Primary Production, also
takes place in aquatic environments (Figure 2). Aquatic plants, plankton, and some
microorganisms use photosynthesis to create OM and pass it further up the food chain.
Written as a simple chemical formula, photosynthesis is:
CO2 + H2O + energy → CH2O + O2
This is simplifying things, so don’t get hung up on minerals, nitrogen, and other nutrients vital to the process. Also, remember plants use photosynthesis to clean CO2 and add oxygen (O2) to the atmosphere. We will come back to the O2 a little later. For now, think of photosynthesis as storing a portion of the sun’s energy as OM. If you don’t believe OM is stored energy, stand in front of logs burning in a fireplace, or try to outrun a grass fire. The heat you feel was once sunlight before photosynthesis converted it to OM.
Fortunately for life on earth, other living creatures can unlock the energy stored
in OM for their own use (Figure 1). When a cow eats grass, she is harvesting the
same energy that the prairie fire releases. Some types of OM contain more energy
than others. It is easier to fatten a cow on grain than grass, because OM in the
grain (starch) is more digestible than OM in grass (cellulose). The cow stores energy
as fat – which is even more energy rich than cellulose or starch.
Figure 1. The Carbon Cycle in Terrestrial Ecosystems.
Figure 2. Trophic Levels and Energy Flow in Farm Ponds.
Oxygen is a key ingredient in unlocking the energy stored in OM. Whether chemical (combustion) or biological (metabolism), O2 is consumed in aerobic conversion of OM to energy. A simplified chemical formula for the process is:
OM + O2 → CO2 + H2O + energy
Notice that this is basically the opposite of photosynthesis. Oxygen is consumed in the aerobic conversion of OM to energy. Fire removes O2 from the atmosphere. If the available O2 is consumed, the fire will go out. Likewise, aerobic microorganisms remove dissolved oxygen (DO) from water. Take enough DO from the water, and fish die.
In combustion, energy is released as heat. Living creatures use metabolic energy
from OM to live, grow, and reproduce. They release some of the energy as heat. Warm
blooded creatures use a portion of the metabolic heat to maintain an elevated body
temperature.
Not all life on earth requires oxygen to live. Anaerobic organisms, organisms that
live in the absence of O2, also tap into the energy stored in OM. Anaerobic digestion is an industrial process
that harnesses energy from OM conversion. A simplified chemical formula for anaerobic
digestion is:
OM + heat → CH4 + CO2 + H2O + energy
Notice that energy in the form of heat appears on the left hand side of the formula. Anaerobic organisms need warm temperatures (20 C and above) to make the process work efficiently. Some of the energy released from OM is stored as CH4 (Methane). Methane is a flammable gas, and we can release its stored energy through combustion:
CH4 + O2 → CO2 + 2H2O + heat
So, if a digester uses heat from burning methane, and we combine the metabolism and combustion parts of digestion, we end up with the same equation as aerobic conversion to energy:
OM + O2 → CO2 + H2O + energy
Organic Matter in the Environment
Soil Environment
The OM content of soil affects moisture holding capacity, nutrient holding capacity,
and particle aggregation. Soil OM supplies nutrients to the soil environment. Think
of soil OM as a pool. The OM pool is constantly being built up and broken down through
chemical and biological action. Decomposition of the pool releases CO2 and plant nutrients.
Manure is sometimes given the euphemism “organic nutrients.” This is because land
application of manure adds both OM and nutrients to the soil. Plant materials (in
particular plant roots) also add to the soil OM pool. Adding inorganic nutrients
(chemical fertilizer) increases soil OM by increasing plant growth.
Soil microorganisms use the energy as well as the nutrients stored in OM to reproduce
and grow. When OM is added to the soil, soil microorganisms respond to the input of
energy by growing and reproducing. If the soil OM pool contains more nutrients than
the microorganisms need, nutrients are released for plant uptake. If the soil is deficient
in nutrients, microorganisms will either not grow or they will take nutrients from
the soil to digest the OM.
The process of microorganisms removing nutrients from the soil is called immobilization.
Immobilization’s effect on soil fertility is usually temporary, but can be devastating
to plant growth. When a high carbon, low nutrient material such as wheat straw or
saw dust is added to the soil, soil microorganisms remove nutrients from the soil
in order to digest the high carbon material. They are basically robbing nutrients
from plants. Eventually, the microorganisms use up the available digestible energy,
die, and release the nutrients stored in their bodies to the soil.
Since nitrogen is the most limiting nutrient for plant growth, the amount of organic
matter to nutrients is expressed as the ratio of carbon to nitrogen, or C:N ratio.
As a general rule of thumb: materials with C:N < 20 release nutrients to soil as
soon as they are added; materials with C:N > 20 immobilize soil nutrients for some
time after they are added.
The nutrient and moisture holding ability of soil OM is related to the amount of nearly
completely decomposed material or humus in the pool. The other benefits of organic
matter, aggregation and release of nutrients, are related to the actively decomposing
portion of the pool. Healthy soils require a constant replenishing of the OM pools
to remain productive.
Aqueous Environments
The oxygen supply is much more difficult to maintain in aqueous compared to terrestrial
or soil environments. Oxygen must dissolve into water before it can be useful. If
animals or microorganisms use DO faster than it can be replaced, O2 is depleted and aerobic organisms die.
One way DO is depleted occurs when excess plant nutrients in the water cause the primary
producers (algae, plankton, aquatic plants) to flourish. During the day, primary
producers pump O2 into the water, at night they remove O2. If nighttime removal outpaces daytime replenishment, DO is depleted. This process,
called eutrophication, takes place in lakes, reservoirs, and estuaries, often far
downstream from where the nutrients were introduced.
A second method of O2 depletion occurs when secondary producers (the decomposers) remove O2 faster than
it can be replaced. Excess OM is usually the cause of this sudden flourishing of
decomposers. Dissolved oxygen depletion due to microbial blooms happens close to
the source of OM addition. This is why the OM content is usually the limiting factor
of wastewater discharge to streams.
Treatment Systems
You have already seen how methane production in digesters is directly related to OM
content of waste. The OM content of wastewater is also important in aerobic liquid
treatment systems such as lagoons and sewage treatment plants. Think of these as
souped-up farm ponds. Aerobic life depends on adequate levels of DO. The amount
of DO added through photosynthesis, natural aeration, or mechanical aeration must
closely match the OM removed from the system. A compost pile is a solid aerobic treatment
system. As in liquid treatment systems, O2 must be added by turning or blowing air
through the pile to match the rate of OM reduction.
A compost pile is also a good example of the energy stored in OM. Because the pile
provides insulation, some of the metabolic heat released during the decomposition
of OM is trapped in the pile. As a result, the pile heats up – sometimes as high
as 180 F before the heat begins to kill off the very organisms that create it. Compost
piles hardly ever get hot enough to spontaneously combust. But, wet hay, carelessly
stacked, will catch fire because of overheated aerobic activity.
Measuring Organic Matter in Byproduct Materials
Organic matter plays a variety of roles in different environments. Likewise, a number of methods have been devised to measure OM content, each with its own uses. We can categorize the various methods of measurement into basic groups: carbon content, loss on ignition, oxygen demand and respiration, and methane production.
Carbon Content
One definition of OM is that it contains carbon. A direct method to determine OM
content is to measure a material’s carbon content. Total Carbon (TC) content is divided into Inorganic Carbon (IC) and Total Organic Carbon (TOC) content. Inorganic carbon is comprised of carbonate, bicarbonate and dissolved CO2. Organic carbon is the carbon atoms in the structure of organic compounds. The
most common method of determining TC is to combust a sample and measure the CO2 released. Before combustion, the IC content is measured by injecting a liquefied
sample into a reaction chamber packed with phosphoric acid coated quartz beads. Under
these acidic conditions, IC is converted to CO2, but the organically bound carbon is not. Total organic carbon is calculated by
subtracting IC from TC.
Loss on Ignition
Another definition of organic versus inorganic material is that OM is combustible.
A sample is first dried to determine the Total Solids (TS) content. The dried sample is then placed in a 550 C furnace for one to two hours.
The material remaining after combustion is Fixed Solids (FS) or Ash content, and the mass that disappears is Volatile Solids (VS) content. Volatile solids analysis is useful because it gives a rough measure of
the total mass of OM in a sample –regardless of its specific chemical makeup. It is
often used to define total mass of OM, and other analyses are used to partition the
VS content into other groups. More information on VS and Solids Testing can be found
in OSU Fact Sheet, BAE-1759, Solids Content of Wastewater and Manure.
Oxygen Demand and Respiration
Organic Matter content of a pollutant is measured indirectly by observing the amount
of oxygen needed to digest it. The two basic measurements of oxygen use are Oxygen Demand and Respiration Rate. Oxygen demand is the total amount of O2 required to aerobically degrade OM. Oxygen demand is further divided into Biochemical
Oxygen Demand (BOD) and Chemical Oxygen Demand (COD). Respiration rate is the rate
at which O2 is removed from the atmosphere or water.
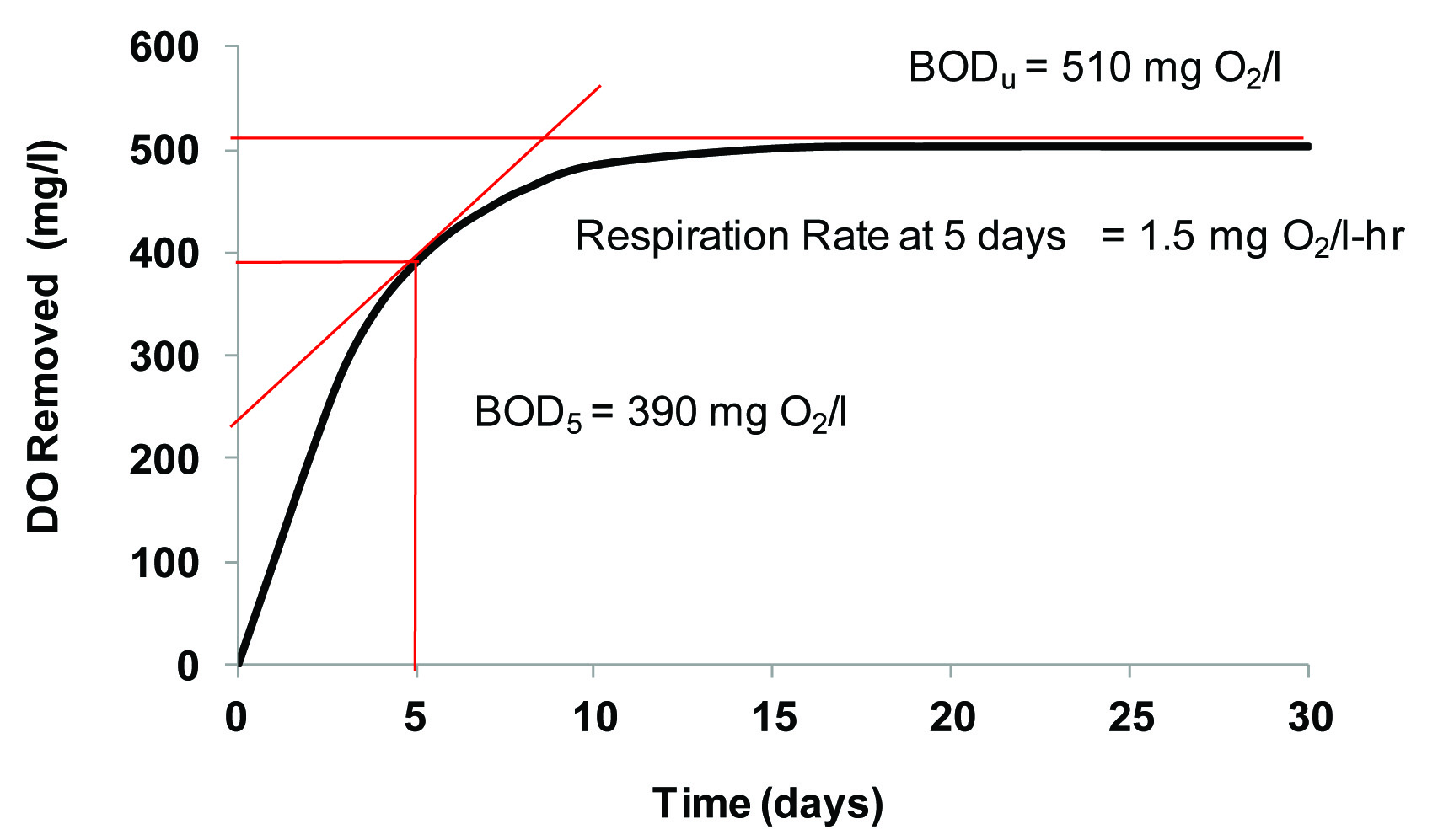
The relationship between oxygen demand and respiration rate is illustrated in Figure
3. Pretend that an OM rich liquid is placed in a sealed container with an infinite
amount of DO. The curve in Figure 3 represents the cumulative amount of DO removed
from the liquid as microorganisms eat the OM. To calculate the amount of O2 removed from the liquid (or demanded by the OM) at any point in time, draw a line
up from the x axis until you reach the curve, then pivot 90 degrees to the left, and
extend the line until you reach the y axis. Oxygen demand is value given on the y
axis, cumulative O2 removed (mg), divided by the volume of liquid (l). To calculate respiration rate,
pick any point on the curve and draw a line with a slope equal to slope of the curve
at that point. Respiration rate is slope (mg/time) divided by the volume (l).
Figure 3. Relationship between Respiration Rate, BOD5, and BODu from DO Removal Curve.
Biochemical oxygen demand is determined by placing a known amount of pollutant in
a sealed flask along with seed organisms and enough pH buffered, nutrient rich liquid
to fill the flask. At the start of the test, the liquid in the container is saturated
with DO. BOD is divided into two values: BOD5 and BODu. Five day BOD or BOD5 is determined by measuring DO removed after five days. The mass of DO removed from
the liquid after five days is divided by the sample volume to give BOD5. Ultimate BOD or BODu is measured by allowing the test to run as long as DO can be removed from the liquid
(generally 30 to 60 days). The DO content of the test flask is usually close to
zero well before the long running BODu test is finished. To keep the microorganisms
alive, the liquid is periodically removed from the flask and reaerated. Dissolved
oxygen is measured using probes.
Nitrogen compounds also have an oxygen demand. For instance, ammonia (NH3) removes DO as it is oxidized to nitrate (NO3). Carbonaceous Biochemical Oxygen Demand or CBOD is determined by adding nitrate inhibitors to the BOD flask before the test begins,
so theoretically, only carbon is oxidized while removing DO.
The Chemical Oxygen Demand or COD is similar to BOD in that it measures the amount of oxygen needed to digest OM, except
that a strong chemical oxidant (usually a mixture of Potassium dichromate and Sulfuric
Acid) is used instead of microorganisms. A dilute sample of pollutant is mixed with
the oxidant in a heated flask and digested for two hours. After two hours, the mass
of dichromate needed to digest the organic matter is measured, and COD is calculated
by dividing O2 equivalents removed by sample volume.
Chemical oxygen demand is related to CBODu in that it measures the maximum amount of O2 used to consume carbonaceous OM. Ammonia is not oxidized in the COD test unless
excess chloride is present in the sample. A little caution is needed, though. A pollutant
will have a COD even though the chemicals in sample may be toxic to microorganisms.
Also, like the BOD tests, the amount of oxidant consumed is related to the time of
digestion. If the analyst does not dilute the sample sufficiently, or the test is
stopped before all chromate is consumed, oxygen demand will be underestimated.
As seen in Figure 3, the Respiration Rate, or slope of the cumulative O2 removal cure, is constantly changing during the digestion of OM. Oxygen removal
rate is great early on in the test as the organisms consume the readily available
energy. Further along, oxygen consumption slows as the food supply dries up. Respiration
rate is a good measure of the energy content of organic matter as it undergoes decomposition.
This is why respiration rate is often reported as the oxygen removal rate per VS concentration
of the material (mg O2/mg VS-min). A byproduct with a high respiration rate contains more digestible energy
than one with a low respiration rate with the same mass of OM.
Respiration rate can be determined in both liquids and solids. There are two basic
methods of determining respiration rate. In both methods, a sample is placed in an
air tight container. In the first method, a known amount of O2 is added and the rate at which CO2 is released is measured. In the second method, a strong base (KOH) is placed in
the sealed container along with the sample. The base removes CO2 as it is released. Pressure drops as the CO2 is absorbed. Respiration rate is determined by measuring the amount of O2 needed to bring pressure in the container back to atmospheric. Newer respirometers
have sensors to detect the pressure drop, and replace the consumed O2 by electrolysis of water. Measuring the electric current required to create the
makeup O2 gives an instantaneous reading of respiration rate.
Methane Production
The set up of a Biochemical Methane Potential or BMP test is similar to a BOD test. A sample is placed in a sealed container with seed organisms and nutrient rich, buffered liquids. Only in a BMP test, the liquid is free of oxygen, and mass of CH4 released over time is measured rather than O2 consumed. Sample size is adjusted relative to seed so that OM will be digested in about 30 days. A word of caution must be given. The BMP test determines the potential CH4 released per mass of VS under ideal conditions. The amount of biogas released in an actual digester depends on the conditions present in the digester and the presence or absence of toxic compounds in the material. For more information on the BMP test read OSU Fact Sheet BAE-1762, Anaerobic Digestion of Animal Manures: Methane Production Potential of Waste Materials
Organic Matter Stability
Stability is the measure of how rapidly carbon is altered in OM. Some organic matter is extremely stable. Diamonds and graphite are pure carbon, but a diamond will not change its composition for thousands of years. There are three basic facets of OM stability. If mixed with the soil, will OM take nutrients away from plants? If stacked, will OM heat up? If left on the soil surface, will OM create odors, draw flies, or invite larger animals to feed?
We have already shown how C:N ratio is used to predict immobilization in soil. A
direct method to predict autoheating ability of solid manure or compost is to place
a sufficient quantity of material into an insulated container, add water to bring
moisture content to approximately 50 percent, and measure temperature rise in the
container after one day. A step by step procedure to use this method is given in
OSU Factsheet, BAE-1761, The Icebox Test: an Easy Method to Determine Autoheating
Potential of Compost and Byproduct Materials.
Putrescibility is a measure of a waste’s ability to release noxious odors and attract
flies. The general definition of a non-putrescible waste is that it cannot undergo
“significant biological transformation.” Decomposing animal bodies undergo a huge
transformation from muscle protein to soil organic matter. Composted manure is non-prutescible
because it lies at a much lower energy state. First, the animal removed energy from
the feed before it was excreted. Then, microorganisms removed more energy from the
manure during composting. We generally rely on other indirect measures, such as the
ability of the material to autoheat to determine the putrescibility of wastes. It
is difficult to predict the autoheating potential of OM — especially with liquids.
Therefore, the energy level of OM, as measured as resipiraiton rate, is usually used
to indirectly measure putrescibility.
The Solvita™ compost stability test is a commercial product that uses respiration and ammonia
volatilization rates to estimate stability. The mass of CO2 and NH3 released by a
compost sample in an airtight container is measured using standard color changing
panels. Organic matter stability is determined by consulting standard charts (Figure
4).
Figure 4. Determining Compost Stability from Solvita CO2 and NH3 results (from Woods End Laboratory, 2009)
Using measurements of OM in Waste Management Systems
An awareness of OM, and the measures used to describe OM, can be used to ensure successful operation of waste handling systems.
Say a dairy farmer uses manure to fertilize corn. Naturally, he would measure plant
nutrients (Total N, Total P2O5, and K2O) to determine an application rate to match a yield goal. Adding TC to the analysis
would allow him to calculate C:N, and determine if he should plant immediately following
application, or wait a few months after spreading manure.
A horse farm, wanting to market compost to local nurseries and gardeners, would characterize
their product using C:N, VS:TS ratio, and respiration or Solvita™ tests to guarantee
its quality.
A wastewater treatment plant is required by its permit to test BOD before discharge.
They might also want to measure TOC and respiration rate at point of discharge to
better predict the effect of OM on the receiving stream.
A swine farmer wants to add an anaerobic digester. He can predict the amount of carbon
available for conversion to biogas using the TOC or COD tests. To get a better estimate
of manure’s potential to produce methane; he would use VS and BMP tests.
References
APHA. 1995. Standard Methods for the Examination of Water and Wastewater, 19th ed. Washington, DC: American Public Health Association.
Girma, K., H. Zhang, and W. Roberts. 2009. Building Soil Organic Matter for Sustainable
Organic Crop Production, PSS-2257. Stillwater, OK: Oklahoma Cooperative Extension
Service.
Donahue, R.L., R.W. Miller, J.C. Shickluna. 1977. Soils, an Introduction to Soils
and Plant Grwoth, 4th ed. Englewood Cliffs, NJ: Prentice-Hall Inc.
Reid, G.K. 1961. Ecology of Inland Waters and Estuaries. NY, NY: Van Nostrand Reinhold
Co.
The Science Learning Hub. 2012. Accessed as www.sciencelearn.nz . Hamilton, NZ:
Waikato University.
Warren, J.G. and B. Arnall. 2011. Organic Matter in No-Till Production Systems,
PSS-2267. Stillwater, OK: Oklahoma Cooperative Extension Service.
Woods End Laboratory. 2009. Laboratory Test Interpretation for Biowastes and Composts.
Mt Vernon, ME: Woods End Laboratory.
Doug Hamilton
Waste Management Specialist