Biodiesel Production Techniques
Base Catalysis/Transesterification
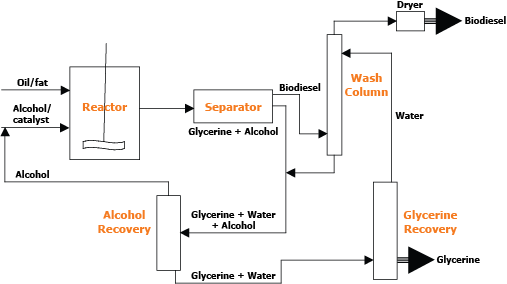
Biodiesel production requires a feedstock (fat or oil) and an alcohol. In most cases, a catalyst also is present. Figure 1 shows a schematic diagram of the unit operations involved in biodiesel production. Depending on the quality of the feedstock, either esterification or transesterification reactions are used for biodiesel production (see FAPC Fact Sheet FAPC-149 Biodiesel Glossary for definition of the terms). Most of the current biodiesel production operations use base catalysis (transesterification). This method works well if the free fatty acid, moisture and phosphorous contents of oil/fat are less than 0.1 percent, less than 0.1 percent and less than 10 ppm, respectively. Typical feedstocks for biodiesel production are soybean, canola/rapeseed, sunflower, cottonseed, palm seed and palm kernel, corn and mustard seed oil. Pork, beef and poultry fat and grease also can be converted to biodiesel. Palm oil and animal fat may have a high free fatty acid content, which causes soap formation that has adverse effects on downstream processing and leads to yield reduction.
Sodium hydroxide (NaOH), potassium hydroxide (KOH) and sodium methoxide (CH3ONa) are the most common catalysts for transesterification. Sodium methylate (sodium methoxide) is more effective than NaOH and KOH as a catalyst, but it is more expensive. Sodium methoxide is sold as a 30 percent solution in methanol for easier handling. Base catalysts are very sensitive to the presence of water and free fatty acids. The amount of sodium methoxide required is 0.3 to 0.5 percent of the weight of the oil. A higher amount of catalyst (0.5 to 1.5 percent of the weight of the oil) is required when NaOH or KOH is used. NaOH and KOH also lead to water formation, which slows the reaction rate and causes soap formation.
Methanol is the most common alcohol used for conversion of fats and oils to biodiesel. Methanol is flammable, so proper handling is required for safety. Detailed information on methanol and biodiesel handling can be found at Biodiesel Community.
Transesterification is a reversible reaction. Thus, excess methanol is required to shift the equilibrium favorably. Table 1 shows approximate amounts of oil, alcohol and catalyst required and the amount biodiesel and glycerine produced through transesterification. Theoretical biodiesel yields for biodiesel soybean and tallow are as follows: 1004.2 kg/1000 kg soybean and 998.1 kg/1000 kg tallow. The amount of methanol in Table 1 is based on 100 percent excess, and the data are for transesterification using sodium methoxide as a catalyst.
Table 1: Approximate reactant and product amounts for transesterification reaction.
| Chemicals/Feedstock/Product | Amount (lbs) |
|---|---|
| Oil/fat | 100 |
| Methanol | 22 |
| Catalyst | 0.3-0.5 |
| Biodiesel | 100 |
| Glycerine | 11 |
| Excess methanol | 11 |
Methanol and oil do not mix well, and poor contact between the oil and methanol reactants means the reaction rate is slow. Vigorous mixing at the beginning of the reaction improves reaction rates. Near the end of the reaction, reduced mixing helps the separation of glycerine, and the reaction would proceed faster in the top layer, which is oil and methanol. At ambient temperature (21 degrees Celsius or 70 degrees Fahrenheit), the reaction takes four to eight hours to complete. The reaction is usually conducted below the boiling point of methanol (60 degrees Celsius or 140 degrees Fahrenheit). At this temperature, the reaction time may vary between 20 minutes to one and a half hours. A higher temperature will decrease reaction times, but this requires use of a pressure vessel because the boiling point of methanol is 65 degrees Celsius or 148 degrees Fahrenheit. The reactor is either sealed or equipped with a condenser to minimize alcohol evaporation during the conversion process. Higher oil conversion rates can be achieved if the production system is set up as a two-step process with two reactors. In such cases, glycerine formed in the first reactor is removed, and the reaction is completed in the second reactor.
Acid Catalysis
If the free fatty acid content of the feedstock is greater than 1 percent, base catalysis is not suitable. There are two approaches for handling high free fatty acid content feedstock.
One way would be to refine the feedstock before base catalysis. Free fatty acids can be removed by chemical neutralization or physical deacidification. Chemical neutralization involves treatment with caustic NaOH or KOH. Soap formed during this process is removed, and the remaining oil is ready for base catalysis. However, some oil is lost during this process. Physical deacidification, or steam stripping, also removes free fatty acids. This process is performed under vacuum and requires steam.
Fats and oils with high free fatty acid content can be converted to biodiesel using acid catalysis, which is the second approach for handling high free fatty acid content feedstock. This technique uses a strong acid. Soap formation is not a problem because there are no alkali metals in the reaction medium. Acid catalysts can be used for transesterification of the triglycerides, but the reaction might take several days to complete. This is too slow for industrial processing.
Acid catalysis also is used for direct esterification of oils with high free fatty acid content or for making esters from soap stock, which is a byproduct of edible oil refining. The esterification of free fatty acids to alcohol esters is relatively fast; it would take about one hour at 60 degrees Celsius to complete the reaction. Water is formed during this reaction. To improve reaction rates, water needs to be removed from the reaction medium by phase separation.
Acid catalysis requires a high alcohol to free fatty acid ratio (20:1 or 40:1 mole ratio) and large amount of catalyst (5-25 percent). Sulfuric acid and phosphoric acid are the most common acid catalysts. The feedstock is sometimes dried to 0.4 percent water and filtered before the reaction. Then, an acid and methanol mixture is added to the feedstock. Once the conversion of the fatty acids to methyl esters has reached equilibrium, the methanol, water and acid mixture is removed by settling or centrifugation. Fresh methanol and base catalyst are added into the remaining oil for transesterification. The rest of the process is the same as base catalysis shown in Figure 1. Reaction times of 10 minutes to 2 hours have been reported.
Figure 1: Typical flow diagram for conversion of oil to biodiesel.
Both transesterification and esterification reactions can be operated either as a batch or continuous process. A batch process is better suited to smaller plants that produce less than 1 million gallons per year and provide operation flexibility. Continuous processing allows use of high-volume separation systems, and therefore increases throughput.
Enzymatic Conversion
There is interest in using lipases for enzymatic catalysis of oils for biodiesel production. The enzymes can be used in solution or immobilized onto a support material, which allows the use of fixed-bed reactors. The reaction can be performed at 35 to 45 degrees Celsius. However, the reaction is very slow, requiring from four to 40 hours. Because of the high cost of the enzymes, this process is not economically feasible for biodiesel production at this time.
Solid Catalyst
The processes discussed above, which also are referred to as homogeneous catalysis, involve utilization of a catalyst that is soluble in alcohol. In these systems the catalyst ends up in the byproducts, and it is not recovered for re-use. There are also solid catalysts that can be used for biodiesel production. This process, which is referred to as heterogeneous catalysis, utilizes fixed-bed reactors, and the catalyst stays in the reactor and is used for an extended time. Sulfonic resins such as Nafion® NR50, sulphated zirconia (SZ) and tungstated zirconia (WZ) have sufficient acid site strength to catalyze biodiesel-forming transesterification reactions as efficiently as sulfuric acid. Alkaline earth metal oxides, various alkaline metal compounds supported on alumina or zeolite can catalyze transesterification reactions. Information about a commercial system that uses a solid catalyst can be found at www.axens.net. In general, heterogeneous catalysis systems are designed for continuous operation and produce high-purity glycerine (greater than 98 percent). The product, fatty acid esters, does not require water washing, and yields are generally high. It also has been reported that catalyst requirements per ton of biodiesel for heterogeneous catalysis are much lower than for other processes. However, these systems operate under high temperature and pressure.
Non-Catalytic Conversion Techniques
Due to poor methanol and oil miscibility, conversion of oil to biodiesel is a very slow reaction. Use of a co-solvent that is soluble in both methanol and oil may improve reaction rates. The BIOX Process (www.bioxcorp.com) uses either tetrahydrofuran (THF) or MTBE-methyl tert-butyl ether as a co-solvent to generate a one-phase system. In the presence of a co-solvent, the reaction is 95 percent complete in 10 minutes at ambient temperatures and does not require a catalyst. THF has a boiling point very close to that of methanol. The excess methanol and co-solvent are recovered in a single step after the reaction is complete. Co-solvents that are subject to the hazardous and/or toxic air Environmental Protection Agency (EPA) list for air pollutants must be completely removed from the biodiesel and its byproducts (glycerine and methanol). Emissions must be tightly controlled, and processing equipment must be “leak proof.”
The second non-catalytic approach utilizes methanol at very high temperature and pressure (350 to 400 degrees Celsius and greater than 80 atm or 1200 psi) to convert oil to biodiesel. This process requires a high alcohol to oil ratio (42:1 mole ratio). The reaction is complete in about three to five minutes. The process requires high pressure vessels which can be quite expensive. The energy consumption also is higher than the conventional processes. The reaction must be quenched very rapidly so the products do not decompose.
Downstream Processing
Biodiesel will be in a mixture of excess methanol, catalyst and glycerine after the completion of the oil conversion reaction. As a rule of thumb difference, in specific gravity of 0.1 in a mixture of compounds will result in phase separation by gravity. As can be seen in Table 2, gravity separation is suitable to recover biodiesel from the process byproducts (glycerine and methanol). However, impurities in the feedstock may cause emulsion formation, which interferes with phase separation. Saturated salt (sodium chloride) or centrifugation breaks the emulsion and speeds up the phase separation.
Table 2: Specific gravity of the compounds used and produced during biodiesel production.
| Compound | Specific Gravity |
|---|---|
| Methanol | 79 |
| Biodiesel | 88 |
| Soybean Oil | 92 |
| Catalyst | 97 |
| Glycerine | 1.28 |
A good conversion reaction will require excess methanol, but the amount of methanol in the system has to be minimized for good phase separation. Glycerine and methanol can be further purified by distillation. Resins or adsorbents have been used to purify biodiesel to eliminate water wash. The residual methanol in biodiesel still needs to be evaporated. Examples of some of the commercial adsorbents and ion exchange resins can be found at Dupont.
Nurhan Dunford
FAPC Oil/Oilseed Specialist