Biodiesel Glossary
- Jump To:
- Acid Number
- ASTM International
- Biodiesel
- Biodiesel Blends
- BQ-9000
- Catalyst
- Cetane Number
- Cloud Point
- Cold Filter Plugging Point
- Corrosion Test
- Esterification
- Fat
- Fatty Acid
- Flash Point
- Heterogeneous Catalysis
- Homogenous Catalysis
- Kinematic Viscosity
- Oil
- Pour Point
- Specific Gravity
- Stability
- Transesterification
- Yellow, Brown and Trap Grease
Acid Number
(Acid Value)
Acid number, also referred to as acid value, is a measure of the amount of mineral acids and free fatty acids in a sample. It is expressed as mg KOH required to neutralize 1g of biodiesel. ASTM D664 describes test conditions. The maximum acid number for biodiesel must be no more than 0.8 mg KOH/g. High fuel acidity is linked with corrosion and deposit formation in engines.
ASTM International
ASTM International, originally known as the American Society for Testing and Materials, is one of the largest voluntary standards development organizations in the world. The society is a reliable source for technical standards for materials, products, systems and services. Best in class practices are used for standards development and delivery. ASTM standards are developed by more than 30,000 ASTM members who are technical experts representing producers, users, consumers, government and academia from more than 100 countries. Participation in ASTM International is open to all with a material interest anywhere in the world.
Biodiesel
Biodiesel is a renewable fuel for diesel engines. It is comprised of mono-alkyl esters of fatty acids which can be derived from vegetable oils, animal fats and algae. Biodiesel is defined in the European Union in the technical regulation EN 14214 or in the United States in ASTM D6751-02. Government regulations define biodiesel as Fatty Acid Methyl Esters (FAME), which is the result of reaction of fatty acids with methyl alcohol. There is some debate to modify the definition to include other products, such as Fatty Acid Ethyl Esters (FAEE), where the methanol can be replaced by bioethanol, giving a product that is fully biological. Biodiesel is an oxygenated fuel. It may be blended with petroleum diesel or used in pure form.
Biodiesel Blends
Biodiesel can be used alone (B100) or blended with petroleum diesel in any proportion. The most popular biodiesel blend is B20 (20 percent biodiesel), which is in compliance with Energy Policy Act of 1992. The American Society of Testing and Materials (ASTM) has approved a standard for biodiesel when used in blends at 20 percent by volume or lower: ASTM D6751 Standard Specification for Biodiesel Fuel Blend Stock for Distillate Fuels. Any diesel engine can operate on these blends with few or no modifications. Blends of up to B20 can meet the ASTM D975 standard. When used in low-level blends of 5 percent biodiesel (B5) or below, biodiesel functions the same as petroleum diesel. When B20 is used, a 1 – 2 percent decrease in power, torque and fuel economy may be experienced; however, these changes are usually not noticeable by the user. Each percent of biodiesel in petroleum diesel increases the cloud point (defined later) by about 0.15°C (1°F/4% biodiesel).
BQ-9000
BQ-9000, or the National Biodiesel Accreditation Program, is a voluntary program for the accreditation of producers and marketers of biodiesel fuel. The program is a combination of the ASTM standard for biodiesel (ASTM D6751) and a quality systems program that includes storage, sampling, testing, blending, shipping, distribution and fuel management practices. BQ-9000 is open to any biodiesel manufacturer, marketer or distributor of biodiesel and biodiesel blends in the United States and Canada. BQ-9000 helps companies improve their fuel testing and greatly reduce any chance of producing or distributing inadequate fuel.
Catalyst
A catalyst is a substance that changes – usually by increasing – the rate of a reaction without itself undergoing any permanent chemical changes. Strong acids, bases and enzymes are used as catalysts for biodiesel production.
Cetane Number
The Cetane Number (CN) is a measure of ignition properties of a diesel fuel. The fuel quality test ASTM D613-05 determines the rating of diesel fuel in terms of an arbitrary scale of CN using a standard single-cylinder, four-stroke cycle, variable compression ratio, indirect-injected engine. CN relates to the delay between when fuel is injected into the cylinder and when ignition occurs. Cetane, a long-chain hydrocarbon with 16 carbon atoms, does not require a pilot flame or an ignition spark and ignites well under high temperature and high pressure conditions. Therefore, it is the ideal fuel for diesel engines. Cetane was assigned a CN of 100 and used as the reference fuel. The higher the CN of a diesel fuel, the better the ignition and combustion. Petroleum-based diesel fuels have a CN of 50 – 52, and the addition of ignition accelerators can increase the range to 53 – 54. Biodiesel compares well with these fuels with regard to its ignition qualities. The CN of biodiesel is directly related to its fatty acid composition. The longer the fatty acid carbon chains and the more saturated the molecules, the higher the CN. Examples of CN for biodiesel (methyl esters) derived from vegetable oils are as follows: peanut 54, soybean 45, palm 62, sunflower 49. Biodiesel standards require a minimum CN of 47. This easily complies with the requirements of engine manufacturers for high-quality fuel with good ignition qualities without the need for additives.
Cloud Point
The cloud point is the temperature at which the smallest observable cluster of hydrocarbon crystals first occurs upon cooling under defined conditions. The standard test, ASTMD 2500-05, describes cloud point determination for diesel fuels. A specific value for the cloud point requirements of diesel fuel has not been set. However, it is suggested the cloud point be no more than 6°C higher than the 10th percentile minimum ambient temperature for the month the fuel will be used. The 10th percentile temperature corresponds to the minimum temperature that would be reached no more than 3 days out of 30 for the month. For example, 10th percentile temperature for the month of December is -12°C in Oklahoma. At low temperatures, biodiesel will gel or crystallize into a solid mass that cannot be filtered or pumped. The engine cannot run at these temperatures. Typical cloud points for biodiesel (methyl esters) derived from vegetable oils are as follows: peanut 5°C, soybean 1°C, palm 13°C, sunflower 1°C.
Cold Filter Plugging Point
The low-temperature performance of diesel fuels also is evaluated with the Cold Filter Plugging Point (CFPP) tests. The method is published as a European standard, ENII6:1981, and it is a national standard in various countries around the world. The CFPP measures the highest temperature at which wax separating out of a sample can stop or seriously reduce the flow of fuel through a standard fuel filter under standard test conditions. The CFPP does not correlate well with low-temperature performance of North American fuels in North American equipment, and it is not included in the ASTM Book of Standards.
Corrosion Test
Some compounds in diesel fuel, especially sulfur compounds and residual catalyst, can be corrosive. Corrosion is readily caused by copper compounds. Hence, copper is used as an indicator of the tendency of the fuel to cause corrosion. ASTM D130-04 uses polished copper strips soaked in the fuel to characterize the tendency to corrode metals.
Esterification
Carboxylic acids (fatty acids) react readily with alcohols in the presence of a catalyst (mineral acids) to yield compounds called esters. The process is called esterification.
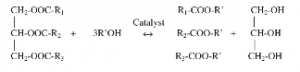
Oil or fat used in alkaline transesterification reactions should contain no more than 1 percent free fatty acids (FFA). Low-quality feedstocks often contain significant quantities of free fatty acids and water, which make them unsuitable for homogeneous alkaline-catalyzed processes. Neutralization of FFAs can be carried out by the addition of excess alkali, but this leads to the formation of soaps and post-reaction separation problems. Thus, a preferred pretreatment process for used cooking oils is an esterification process that commonly uses a strong liquid acid catalyst, such as sulfuric acid.
R-COOH + R1-OH ⇆ R-COO-R1 + H2O
Fatty acid Alcohol Catalyst Ester/Biodiesel Water
Fat
Fats are a mixture of triacylglycerides and usually solid under ambient conditions.
Fatty Acid
Fatty acid is an organic compound consisting of a hydrocarbon chain and a carboxylic acid group. Long chain fatty acids (more than 8-10 carbon atom on the hydrocarbon chain) are most commonly present in oils and fats. These fatty acids usually have an even number of carbon atoms and unbranched chains.
Fatty acids may be “saturated,” which means there is no double bonds on the hydrocarbon chain, or “unsaturated” with one or more double bonds. If a fatty acid contains two or more double bond in the structure, it is “polyunsaturated.”
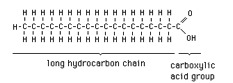
(Unesterfied Fatty Acids)
Free fatty acids are not bound or attached to other molecules such as glycerol. The unesterified fatty acids may come from the breakdown of a triglyceride into its components (fatty acids and glycerol). They also may arise from incomplete conversion or hydrolysis during storage of feedstock. Free fatty acids are very soluble in biodiesel and can further compromise oxidative stability during storage. Biodiesel with high fatty acid number can increase deposits in the fueling system. Free fatty acids must be removed during edible-oil processing because they reduce the smoke point in frying fats and rapidly oxidize to give rancid flavors. Food-grade oils usually contain less than 0.05 percent free fatty acids.
Flash Point
Flash point is the lowest temperature at which the vapor of a combustible liquid can be made to ignite momentarily in air. The flash point test method is described by ASTM D-93. All No. 2 diesel fuels have high flash points (54°C, minimum; 71°C, typical). According to ASTM regulations, biodiesel must have a flash point minimum of 130°C (266 F), while No. 2 petroleum diesel is required to have a flash point above 52°C (125 F). The U.S. Department of Transportation considers a material with a flash point of 93°C or higher to be nonhazardous. A low flash point in biodiesel can indicate residual methanol remaining from the conversion process. A high flash point can mean the oil conversion reaction has not proceeded to completion. A low flash point in biodiesel can result in premature ignition, causing irregular timing, excessive fuel blow-by into the crankcase oil and excessive emissions. A high flash point can lead to poor ignition, resulting in inconsistent firing and variable engine performance. It also can indicate potential clogging of fuel lines, filters and injector clogging in cold climates.
(Glycerin)
Glycerol, the simplest trihydric alcohol [C3H5(OH)3], is a byproduct of the biodiesel process. It is a water soluble/miscible, colorless, sweet tasting and viscous liquid. In commerce, it is usually called glycerin, sometimes spelled glycerine. In general “glycerol” refers to the pure compound, whereas “glycerin” is used for lower-purity products.
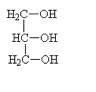
One important property of glycerol is that it is safe for human consumption. Therefore, it is used in foods, syrups, ointments, medicines and cosmetics.
Heterogeneous Catalysis
Heterogeneous catalysis involves the use of a catalyst in a different physical phase than the reactants. Typical examples involve a solid catalyst with liquid or gaseous reactants. The main advantage of a heterogeneous catalyst is that the catalyst is easily separated from the reactants and products. A common approach is to pack a reaction vessel with catalyst particles and stream reactants through the packed bed. Currently, research is being conducted on numerous heterogeneous catalysts for biodiesel production. Axens, a Paris-based refining, petrochemical and natural gas market-focused company, has already developed a commercial biodiesel production technique (Esterfip-H) based on heterogeneous catalysis. This system eliminates water washes, and the product-separation scheme is simple and clean with less effluent to treat and dispose. Furthermore, the reaction byproduct glycerin is 98 percent pure. Esterfip-H requires slightly higher operating temperatures to perform, but the stability of the catalyst and simplified downstream processing reduce conventional biodiesel production costs.
Homogenous Catalysis
Homogeneous catalysis involves the use of a catalyst in the same physical phase as the reactants. The majority of current biodiesel processes use homogeneous catalysts, such as a strong acid like sulfuric or hydrochloric acid or strong base-like sodium or potassium hydroxide or methoxide dissolved in alcohol. Hence, oil, alcohol and catalyst are all in the liquid phase.
Kinematic Viscosity
Viscosity is a measure of a fluid’s resistance to flow. Kinematic viscosity can be obtained by dividing the absolute viscosity of a fluid by its mass density. The greater the viscosity, the less readily the liquid flows. Viscosity decreases strongly with increasing temperature. Biodiesel is more viscous than No. 2 diesel fuel, but only by a small amount. ASTM D445 is a standard test procedure for determining the kinematic viscosity of liquids. According to ASTM standards, kinematic viscosity is measured at 40°C and should be between 1.9 and 6.0 mm2/s.
Oil
Oils are liquid at atmospheric conditions. More than 90 percent of oils consist of triacylglycerides.
Pour Point
Pour point is the temperature at which the fuel ceases to flow. One drawback of biodiesel is that it has higher cloud and pour points than petroleum diesel, which could lead to engine problems during winter in much of the United States. The pour point always is lower than the cloud point.
Specific Gravity
The specific gravity is defined as the ratio of a given volume of a sample material at 25°C to the weight of the same volume of water at 25°C. Specific gravity is determined by ASTM D-287. No. 2 diesel exhibits a specific gravity of 0.85. Biodiesel specific gravity varies between 0.86 and 0.90 depending on the feedstock used.
Stability
In regards to biodiesel, the term “stability” refers to thermal stability under both hot and cold temperatures, resistance to oxidation, polymerization, water absorption and microbial activity. The main source of instability in biodiesels is unsaturated fatty acid chains. Stability also can be affected by metals and plastics in contact with biodiesel. The presence of water in biodiesel can cause rust formation and corrosion. The presence of water also promotes microbial growth. Water and sediment analysis in biodiesel is described by ASTM D2709. According to ASTM D6751-06, the maximum allowable water and sediment is 0.05 percent by volume. Oxidation stability of diesel fuel is measured by ASTM D2274. Oxidation of biodiesel leads to the formation of hydro peroxides, which can polymerize to form insoluble gums and cause plugging in fuel systems and filters.
Transesterification
Transesterification is the general term used to describe the important class of organic reactions where an ester is transformed into another through interchange of the alkoxy moiety. In biodiesel production process, transesterification refers to the reaction of a lipid (triacylglyceride) with an alcohol (short-chain alcohols such as methanol, ethanol or buthanol) to form fatty acid esters (biodiesel) and a byproduct – glycerol.
The reaction is reversible. Usually, an excess of alcohol is used to force the equilibrium to the product side. The stoichiometry mole ratio for the reaction is 3:1 alcohol to lipids. However, in practice, this is usually increased to 6:1 to raise the product yield. If methanol is used in this process, it is called methanolysis. The presence of a catalyst (a strong base or acid enzyme) accelerates the conversion. Most biodiesels are currently synthesized using an alkaline catalyst, because the transesterification reaction by an acid catalyst is much slower.
(Triaclyglyceride)
A triacylglyceride is a compound formed from one unit of glycerol and three units of fatty acids. In simple triacylglycerides, all three fatty acids are identical. In mixed triacylglycerides, two or three different fatty acids may be present. Fats and oils are physical mixtures of many different triacylglycerides.
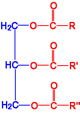
The blue section on the figure represents a glycerol molecule, and the red section is esterified fatty acids to a glycerol molecule. R, R’ and R” symbolize hydrocarbon chains on fatty acids.
Yellow, Brown and Trap Grease
Yellow grease is produced from vegetable oil or animal fat that has been heated and used for cooking. Renderers filter out the solids and heat the spent cooking oil to drive out moisture until it meets industry specifications for yellow grease. Yellow grease is required to have a free fatty acid (FFA) level of less than 15 percent. If the FFA level exceeds 1 percent, it is called brown grease. This grease may be sold at a discount or blended with low FFA material to meet yellow grease specifications.
Trap grease is material that is collected in special traps in restaurants to prevent the grease from entering the sanitary sewer system where it could cause blockages. Many rendering plants will not process trap grease because it is usually contaminated with cleaning agents.
Yellow grease, brown grease and trap grease can be used to produce biodiesel.
Nurhan Dunford
FAPC Oil/Oilseed Specialist
