Anaerobic Digestion: Biogas Utilization and Cleanup
Summary
One method of animal manure disposal is anaerobic digestion (AD). The term anaerobic implies that at least part of the process occurs in the absence of oxygen. Farm AD, or bio-digesters, normally take animal manure and produce combustible gas (“biogas”) and useful solid matter. Most AD units produce a biogas that contains methane and has an energy content approximately 60 percent that of natural gas. Biogas can be used to power engine/generators to make electricity or to provide gas for various heating applications. Biogas could be a fuel for fuel cells also. The use of AD biogas to provide energy for processes on the farm has been known for some time. As natural gas and electricity prices increase, the use of biogas may be a good economic source of renewable energy for the farm. However, for most applications the gas needs to be cleaned or purified prior to use. This Fact Sheet briefly examines some of the AD biogas pre-use cleaning or processing methods.
Biogas Properties
Biogas generated from anaerobic digestion processes is a relatively clean and environmentally friendly renewable fuel. Raw biogas contains approximately 55 percent to 65 percent methane (CH4), 30 percent to 45 percent carbon dioxide (CO2), traces of hydrogen sulfide (H2S) and hydrogen (H2), and saturated water vapor. Biogas is about 20 percent lighter than air and has an ignition temperature in the range of 650 C to 750 C (1,200 F to 1,380 F). It is an odorless and colorless gas that burns with a flame similar to that of natural gas. However, biogas has an energy content of 20 to 26 MJ/m3 (537-700 Btu/ft3) compared to commercial quality natural gas’ energy content of 39 MJ/m3 (1,028 Btu/ft3) [SEAI].
Biogas Utilization
There are a variety of end-uses for biogas. Except for the simplest thermal uses such as some types of heating or odor flaring, biogas needs to be cleaned or processed prior to use. With appropriate cleaning or processing, biogas can be used in all applications that were developed for natural gas. The three basic end-uses for biogas are: production of heat and steam, electricity generation, and as a vehicle fuel. Consequently biogas can potentially be used in:
- Internal Combustion (Piston) Engine – Electrical Power Generation, Mechanical Shaft Power
- Gas Turbine Engine (Large) – Electrical Power Generation, Mechanical Shaft Power
- Microturbine Engine (Small) – Electrical Power Generation
- Stirling Heat Engine – Electrical Power Generation
- Boiler (Steam) Systems
- Hot Water Systems
- Process Heaters (Furnaces)
- Space or Air Heaters
- Gas Fired Chiller – Refrigeration
- Absorption Chiller – Refrigeration
- Combined Heat and Power (CHP) – Large and Small Scale – Electrical Power and Heat
- Fuel Cells – Electrical Power, Some Heat
Conventional gas burners
These are easily adjusted for biogas by simply changing the air-to-gas ratio. The requirements for biogas energy content and preprocessing in gas burners is relatively low, only requiring a gas pressure of 8 to 25 mbar and maintaining H2S levels to below 1,000 ppm to in order to minimize acid condensation. Hydrogen sulfide (H2S) might eventually erode the burners enough to require replacement.
Combined heat and power (CHP)
Internal combustion engines are the most frequently mostly used power plants in AD-CHP applications due to familiarity, cost and ability to simply recover heat. Gas turbines (micro-turbines, 25-100 kW; large turbines, > 100 kW) have comparable efficiencies to spark-ignition engines and can be used for production of both heat and power. The use of biogas in either of these engine systems require removal of both H2S (to below 1,000 ppm) and water vapor.
Fuel cells
These are considered the small-scale power plants of the future for production of power and heat with efficiencies exceeding 60 percent and low emissions. One of the largest digester/fuel cell units is located in Washington State and uses municipal waste. The fuel cell, located at the South Treatment Plant in Renton, WA, can consume about 154,000 ft3 (4,361 m3) of biogas a day to produce up to 1 Megawatt (1,000,000 Watts) of electricity. That’s enough to power 1,000 households, but it’s being used instead for the operation of the plant. Biogas used in fuel cells must go through rigorous purification methods to remove contaminants such as sulfides and siloxanes [Drewitz].
Vehicle fuel
Gas powered vehicles can use biogas as fuel, provided it is upgraded to natural gas quality (i.e., removal of water vapor, CO2 and H2S). Most vehicles in this category have been retro-fitted with a gas tank and a gas supply system in addition to the normal petrol fuel system. Dedicated vehicles (using only biogas) are much more efficient than these retro-fits.
To reach as many consumers as possible, biogas injection into the gas grid is definitely a possibility in the future. However, stringent cleaning standards will have to be in place to avoid contamination of the gas-grid. Upgrading or cleaning methods must thus allow treated biogas to meet such stringent quality standards [Hagen].
Biogas Cleanup or Upgrading
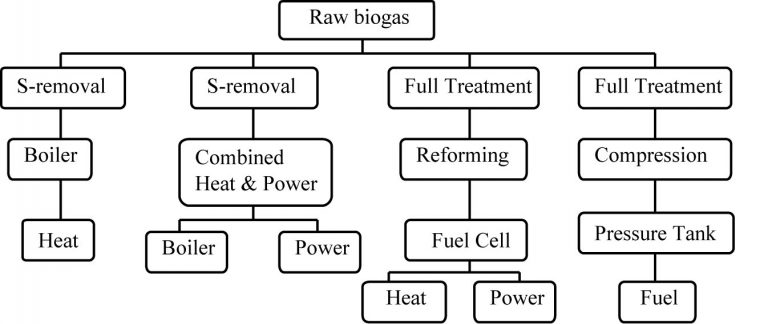
Biogas cleaning is important for two reasons: (i) increasing the heating value of biogas, and (ii) to meet quality requirements for some gas appliances (engines, boilers, fuel cells, vehicles, etc). Desired biogas cleaning or upgrading purposes are summarized in Figure 1 [Ndegwa, Frazier]. Full treatment implies that biogas is cleaned of CO2, H2S, water vapor, and other trace gases, while reforming is conversion of methane to hydrogen.
Figure 1. Alternative biogas utilization and required cleanup.
Heating value
For many of the simpler biogas applications such as heaters or internal combustion engine- generator systems, carbon dioxide (CO2) removal from biogas is not necessary and CO2 simply passes through the burner or engine. For more demanding biogas/engine applications such as vehicles which require higher energy density fuels, CO2 is routinely removed. Removing CO2 increases the heating value and leads to a consistent gas quality similar to the natural gas. Carbon dioxide removal from biogas can be performed economically through absorption or adsorption. Membrane and cryogenic separations are other possible methods.
Pressurized counter-current scrubbing of CO2 and H2S from biogas can be accomplished in water. For removal of CO2 in particular; pH, pressure, and temperatures are critical. High pressures, low temperature, and high pH increases CO2 scrubbing from biogas. Use of Ca(OH)2 solutions can completely remove both CO2 and H2S. Both CO2 and H2S are more soluble in some organic solvents such as polyethyleneglyco and alkanol amines that do not dissolve methane. These organic solvents can thus be used to scrub these gases from biogas even at low pressures. Systems using these kinds of organic solvents can remove CO2 down to 0.5 percent in the biogas. However, use of organic solvents is much more expensive than water based systems.
Adsorption of CO2 on solids such as activated carbon or molecular sieves is possible although is accomplished at high temperatures and pressures. These processes may not be cost-effective because of the associated high temperature and pressure drops. Cryogenic separation is possible because at normal atmospheric pressure, methane has a boiling point of -106 C, whereas CO2 has a boiling point of -78 C. Fractional condensation and distillations at low temperatures can thus separate pure methane in liquid form, which is convenient for transportation. Biogas with up to 97 percent pure methane can be obtained but the process requires high initial and operational investments. Membrane or molecular sieves depend on the difference in permeability of individual gas components through a thin membrane. Membrane separations are quickly gaining traction. Other chemical conversions are technically viable but their economics (Table 1) may be poor for practical biogas-cleaning [Ndegwa, Frazier].
Table 1. Examples of relative costs of different scrubber technologies from industry survey. Some costs depend on scale of operation. (Chen, et. al.)
| Upgrade Method | Contaminants | Cost to Upgrade (Clean) Biogas ($/1,000 ft3) |
|---|---|---|
| Biological | H2S | 1.86 |
| Iron Oxide (Sulfa Treat®) | H2S | 0.797 |
| Iron Oxide (Sulphur Rite®) | H2S | 1.49 |
| Membrane (SeparexTM®) | CO2, H2O | 2.13 |
| Water Scrubber | CO2 | 0.381 |
| KOH-Activated Carbon | H2S | 0.455 |
| Selexol® Physical Absorbent | H2S, CO2, H2O | 5.08 |
Water Vapor Removal
Straight from the digester, biogas will generally be saturated with water vapor. Besides reducing the energy value of biogas, water can reacts with H2S to create ionic hydrogen and/or sulfuric acid which is corrosive to metals. Refrigeration or sensible pipe-work (thermal heat piping) design can condense and remove the water. The biogas is normally compressed before cooling to achieve high dew points and precipitate out water [Wellinger]. Alternative water vapor removal mechanisms include adsorption on: (i) silica gel and aluminum oxide (Al2O3) at low dew points, (ii) glycol and hygroscopic salts at elevated temperatures, and (iii) molecular sieves.
Removal of H2S
Hydrogen sulfide in biogas should be removed for all processes except in the most simple burner applications. Hydrogen sulfide in combination with water vapor in raw biogas can form sulfuric acid (H2SO4), which is very corrosive to engine components. The short term exposure limits for H2S is 15 ppm (MSDS). At concentrations above 100 parts per million by volume (ppmv), hydrogen sulfide is considered very toxic. There are a variety of H2S removal methods, some of which are discussed below.
Activated carbon can be used to remove both H2S and CO2. Activated carbon catalytically converts H2S to elemental sulfur. Hydrogen sulfide can also be scrubbed from biogas in either: NaOH, water, or iron salt solutions. A simple and relatively inexpensive process is to dose a stream of biogas with air injection (O2), which oxidizes H2S to elemental sulfur and water. Oxygen dosing can reduce the H2S in biogas to approximately 50ppm.
Iron oxide also removes H2S as iron sulfide. This is a well known method called the “iron sponge” and is widely used in petrochemical operations. This method can be somewhat sensitive to high water vapor content of the biogas. The ability to remove biogas H2S down to fuel cell levels (<1ppm) with the iron sponge method is currently under investigation at the Department Biosystems and Agricultural Engineering at Oklahoma State University.
Membrane filtration methods have also been developed for H2S removal from gas streams. The gas stream is heated and passed through various size membranes that allow some gases to permeate. The gases that do not permeate are usually the fuel content gases such as methane. The permeating gases are more typically the “wastes” such as H2S, CO2 etc.
Biofiltration (including Thermophilic bacteria) uses biological action to reduce H2S levels. Some anaerobic bacteria can metabolize various compounds including sulfur. Gases containing these unwanted compounds can be passed over and through media containing the bacteria. The bacteria will subsequently remove the compounds from the gas. Some bacteria have been found that can metabolize greater quantities of material at higher temperatures (Thermophilic). One such bacterium, thermochromatium tepidum, can metabolize H2S to produce elemental sulfur [Ryu, et. al.].
Conclusion
Biogas produced from animal manure is an option for producing thermal or electrical energy on the farm. The resulting gas produced by the anaerobic digestion may require some purification prior to use. Understanding the needed purity level of gas can help the producer select the most economical purification system for their end-use requirements. Some end-uses such as heating may not need gas purification. Technologies such as fuel cells will probably require extensive H2S removal. Fuel cells typically cannot tolerate H2S levels of more than 1 part per million and can be easily ruined. [EMG International, NYS ERDA]
For helpful information on biogas production and associated technologies see the U.S. Department of Energy.
References
Chen, P, A. Overholt, B. Rutledge, J. Tomic, 2010. Economic Assessment of Biogas and Biomethane Production from Manure, White Paper for: CALSTART, Pasadena, California.
Drewitz, M., P.Goodrich. 2005. Minnesota dairy runs hydrogen fuel cell on biogas.
EMG International, Inc. 2007. Biogas Clean-Up Technologies. Presentation to NYS ERDA Innovations in Agriculture.
MSDS (Material Safety Data Sheet) for Hydrogen Sulfide, Philips 66 H2S MSDS, August 15, 1995.
Ndegwa , P., R. Frazier, 2010. Biogas Utilization and Cleanup. Cooperative Extension System, eXTension© Foundation.
Ryu, Hee-Wook, Sun-Kyung Yoo, Jung Min Choi, Kyung-Suk Cho, Daniel K. Cha. 2009. Thermophilic biofiltration of H2S and isolation of a thermophilic and heterotrophic H2S-degrading bacterium, Bacillus sp. TSO3. Journal of Hazardous Materials 168: 501-506.
Wellinger, A., A. Lindberg. 2005. Biogas Upgrading and Utilization. Task 24: Energy From Biological Conversion of Organic Waste. IEA Bioenergy.
R. Scott Frazier
Assistant Professor, Energy Management Specialist
Douglas Hamilton
Associate Professor, Extension Waste Management Specialist
Pius M. Ndegwa
Associate Professor, Washington State University