IPM- Scouting and Monitoring for Pests in Commercial Greenhouses
Environmental issues, restricted labeling of pesticides, pest resistance, and public opinion are all concerns causing the commercial grower or retailer to seek alternative forms of pest control. Integrated pest management (IPM) is a holistic approach to pest control in which multiple practices are implemented throughout the entire production period of the crop. Some of these practices are prophylactic—good sanitation and sound cultural practices. Other practices are implemented as control methods—applying less toxic and/or environmentally sensitive pesticides and introducing biological control agents, as well as the judicious use of traditional pesticides.
Crop monitoring is the foundation of an IPM program. Crop monitoring provides heightened awareness of pest presence, activity, and control. It addresses the real needs of the crop, reduces pesticide use by eliminating unnecessary, routine applications, and assures that pesticides are applied at the proper life-cycle stage to insure effectiveness.
Prerequisites
A successful IPM program depends upon a successful monitoring program. There are several prerequisites which must be satisfied to accomplish this. An implementor of an IPM program must:
- Commit enough time and sufficient money to expand pest control practices from total dependence on pesticides to a truly integrated system.
- Establish an IPM team and decide who is going to make pest management decisions.
- Provide team members with the proper equipment including: 10X hand lens or headset with a magnifier, colored tape or flags, books with pictures for proper identification of pests, maps of greenhouses, and access to a diagnostic laboratory (such as the Plant Disease and Insect Diagnostic Laboratory at OSU).
- Divide the greenhouses into logical units and then make maps of these units so all members of the IPM team can communicate pest and crop information uniformly.
- Provide adequate training to team members to allow them to identify pests in all life cycles.
Scouting Methods
The primary goals of monitoring are to locate and identify insect, mite and disease problems, and to observe changes in the severity of infestation. These are accomplished by random plant inspections throughout the production area and by the use of sticky traps and indicator plants.
Random plant inspections should be performed weekly or, preferably, twice weekly during the entire production season. Scouting procedures should be as routine as possible. Monitors should establish a pattern that will cover all areas of the greenhouse and follow the same pattern every time. Scouting must be intensive; the more plants monitored the better. Scouting should start from a major doorway. This is often the location where disease and pest problems begin. Special attention should be paid to plants around any openings in the greenhouse, especially those plants on the outside rows of benches.
Monitors should walk every aisle and move from bench to bench in a snake-like or zig-zag pattern. At least 10 minutes should be spent inspecting 20 or more plants from every 1,000 square feet of production area. The number and size of plants will affect the scouting pattern, as well as the location and size of the benches in the greenhouse. At least three plants on every bench should be inspected, from the edge, the middle and as far into the bench as can be reached. Individual plants should be chosen at random and inspection should include checking for insects, mites, or disease symptoms. Inspection is begun at the bottom of the plant and proceeds upwards, from older leaves to younger leaves to new growth. Special attention should be paid to buds and blooms. Pots should be tipped sideways for inspection of the underside of the leaves where many pests reside. Root examinations should be performed on crops that are highly susceptible to root disease by inverting and removing the pot. Hanging baskets must also be inspected. Since most pests that attack greenhouse crops do not distribute themselves evenly throughout the crop, one should never assume to know exactly where the pests are, or a serious misjudgment could occur and an entire infestation overlooked.
In addition to random plant monitoring, a daily inspection of indicator plants and
sticky traps is ideal. The first diseased or pest-infested plant found on a bench
becomes an indicator plant. This plant is marked with a stake or in some manner that
allows the employee to check the same plant daily. Checking the same plant daily allows
for an ongoing close examination of pest populations or symptoms as they spread to
surrounding plants. The scout can also follow the development of a pest problem, noting
the rate at which the life cycle is progressing. Tracking the development rate provides
the manager with necessary information regarding the best time for pest control measures,
if necessary. Indicator plants can also be used to check if treatments were effective.
A highly susceptible host plant is an excellent indicator plant. Grown among the commercial
crop, it is the first plant to become infested or diseased and helps simplify crop
monitoring. Some highly susceptible host plants and the pests they attract are as
follows:
- Whiteflies—tomato, lantana, gerbera daisy, poinsettia, and eggplant.
- Spider mites—marigolds and roses.
- Aphids—sweet peppers and fuschias.
- Thrips—petunias and impatiens.
- Tomato Spotted Wilt Virus—petunias and gloxinias.
- Necrotic Spotted Wilt Virus—impatiens.
While indicator plants are useful as monitoring devices, care must be taken so that they do not become sources of reinfestation.
Another method of monitoring a greenhouse for insect pests is the use of sticky traps. The traps come in two colors, a bright yellow and a medium blue. Most greenhouses use the yellow traps which attract flying aphids, fungus gnats, whiteflies, leafminers, thrips, and others. Blue sticky traps are used to attract mainly thrips. These brightly colored cards are covered with a sticky substance that traps the insects. These may be purchased pre-made or materials may be purchased separately and the traps made by hand, which is more economical. When using only one color, yellow traps should be chosen because of their universal attractiveness.
Sticky traps should be placed throughout the greenhouse. They should be placed in a grid-like fashion, at least one card per 1,000 square feet of production area. More traps per square foot of production area are beneficial. They are placed just above the plant canopy to 16 inches above the crop. Sticky traps should be placed in the same position every time. This allows for accurately determining the increase or decrease of pest populations as the production season ensues. Sticky traps should be checked at least once a week. Most growers prefer to check them every three days. Daily monitoring of the traps is recommended if time and personnel permit.
Time counting insects on sticky traps may be reduced by counting the insects within an one inch wide vertical column on the trap. Aphids and thrips tend to be caught on the bottom half of the traps. Leafminers are caught more often along the top, and leafminer wasps and whiteflies tend to be spread uniformly over the trap. Aphids tend to be caught in the middle vertical columns. Since insects are not distributed evenly horizontally across the trap, columns counted should be vertical towards the middle of the trap.
To identify insects on the sticky traps, a 5X to 10X power hand lens will be necessary.
When handling the sticky traps, it is beneficial to have some waterless hand cleaner
nearby. The adhesive used on the traps is a polybutene-naphtha rubber polymer that
remains viscous for long periods. It is difficult to examine the traps without getting
adhesive on the hands. The following is a list of characteristics to help in the identification
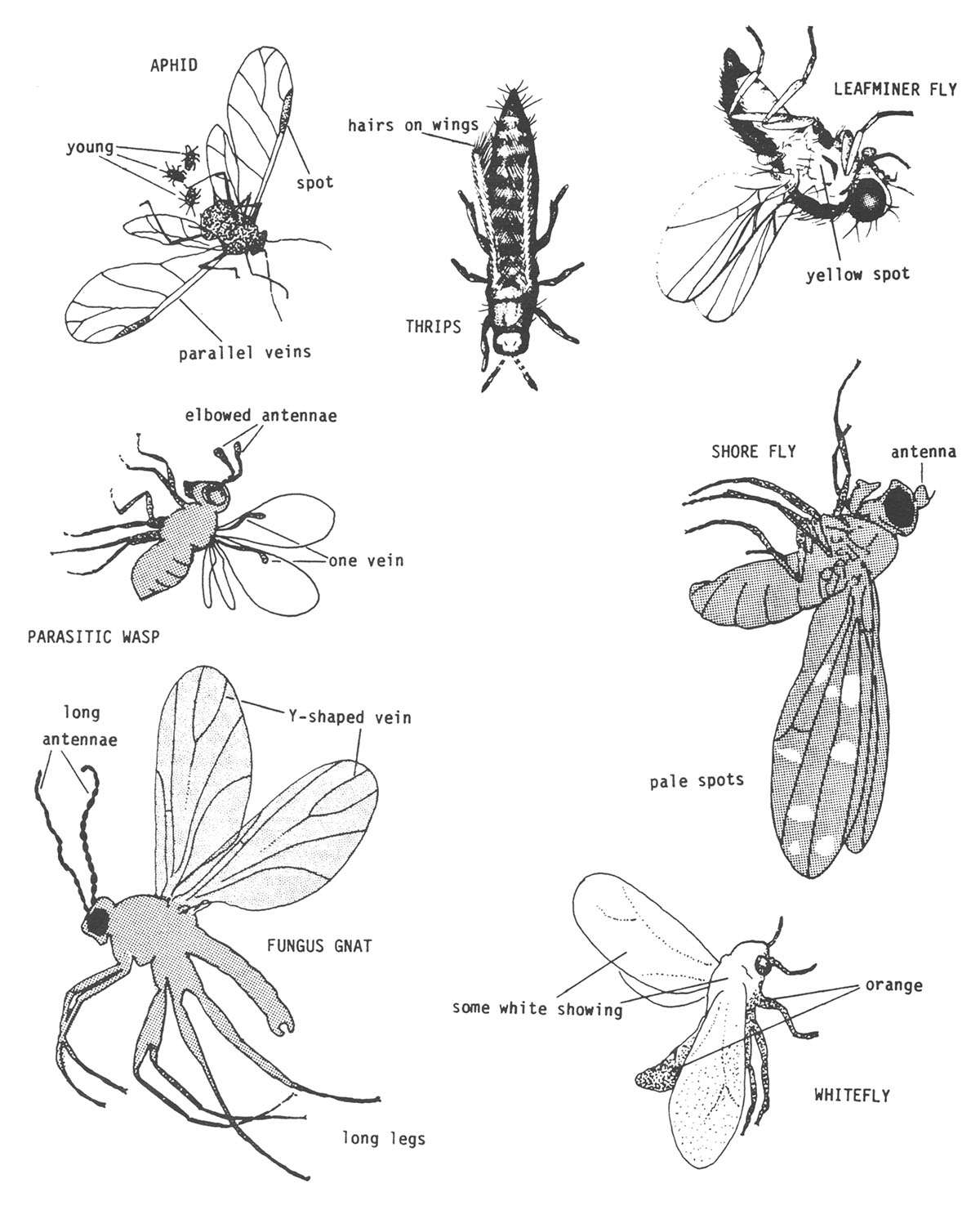
of insects on sticky traps (Figure 1):
- Aphids. The wings of aphids often settle symmetrically into the adhesive to either side of the body. They sometimes give birth to one to five nymphs before they die. The front wings usually have two parallel veins close to the front edge. These veins end at a dark, skinny spot on the front edge. The legs and antennae seem to be long and skinny.
- Fungus Gnats. These are small, dark, mosquito-like insects with gray wings. The wing has a distinct, Y-shaped vein at the tip. They have long, skinny legs and antennae.
- Leafminer Flies. Unless the specimen is totally embedded in the adhesive, it is possible to see a conspicuous yellow spot on each side. They have short antennae and moderately long legs.
- Parasitic Wasps. These usually have antennae with elbows like an ant, and the forewings have only one vein which zigs toward the front margin and zags away. Usually parasitic wasps are more pointed at the rear than shore flies.
- Shore Flies. These are the largest common fly usually found on sticky traps. They have pale spots on the wings, the antennae are short and the legs are moderately long. Care should be taken when identifying, since shore flies are often confused with fungus gnats.
- Thrips. These are the tiniest insects found in any numbers on the trap. Most appear spindle-shaped with the wings protruding neatly at the rear. Hairs line the edges of the wings. Often, the stocky antennae protrude in a V-shape at the front.
- Whiteflies. Whiteflies lose their white, waxy bloom as they are entrapped by the adhesive. They are only a little larger than thrips and show up orange on the traps. Usually enough of a wing or leg protrudes above the adhesive so that the white bloom reveals the identity.
Figure 1. Courtesy of Dr. James R. Baker, Extension Entomologist, North Carolina State University, Raleigh.
Record Keeping
Without proper records, scouting will be ineffective. IPM programs depend upon keeping detailed records. Incoming plant material inspections, random and indicator plant inspections, sticky trap information, and crop treatments must be recorded. All production inputs must be noted concisely and accurately (Tables 1 and 2). Managers trying to diagnose a problem without records are at a disadvantage and will overlook potential causes of the problem. Maps of the greenhouses showing where benches, sticky traps, and indicator plants are located should be maintained. Disease, mite, and insect infestations can be penciled in on these maps, and movement of the infestation can be monitored. As the season progresses, pest trends develop, and a direction for pest management decisions will be seen.
Weekly summaries should be compiled when a scout is finished with the week’s monitoring (Table 3). The information is itemized for each greenhouse, according to the pests detected, the counts, and any unusual circumstances found in the greenhouse.
Detailed records of any pesticide application should be kept to compare with previous
records to see if fewer applications have been made or if a less toxic chemical has
been substituted. These records should include the following information (Table 4):
- Date of spray application.
- Name, classification, amount of active ingredient, and registration number.
- Amount of material and water mixed for the application.
- How much of the pesticide was actually applied.
- Where the pesticide was applied.
- Square feet or number of pots treated.
- Type of application method (wet spray, fog, etc.).
- Applicator’s name.
- Labor hours.
IPM has the potential to save growers money on chemical costs; however, scouting will cause an increase in labor cost.
Implementing an IPM program takes time and patience. It also requires changes in production strategy. More labor hours must be invested into the production of a crop, and detailed records must be kept. However, there are rewards. Growers over time can determine their own economic threshold for any given insect, related pest, or disease. Fewer pesticide applications mean less cost to the manager, less hazards to the worker, and less potential damage to the environment. It will also decrease the likelihood that pests will develop a resistance to a traditional pesticide, which will give the manager one more weapon in his/her arsenal against pests.
Mike Schnelle
Extension Ornamentals/Floriculture Specialist
Eric Rebek
Extension Entomologist